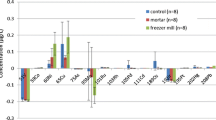
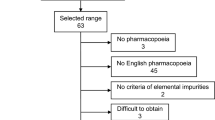
One element of ensuring the quality and safety of medicines consists of normalizing and controlling elemental impurities (heavy metals). The sources and causes of the presence of elemental impurities (heavy metals) in medicines are discussed. Changes in approaches to assessment of elemental impurities in medicines in the State Pharmacopeia and leading non-Russian pharmacopeias are discussed. A risk-oriented approach to assessing elemental impurities used in international practice is presented.
Similar content being viewed by others
References
Quick reference: Analysis of Elemental Impurities in Certified Pharmaceuticals Laboratories [in Russian], Agilent Technologies; [online resource], https: // www.campilab.by / file / 1145991 – 0436ru.pdf / 5991 – 0436RU.pdf.
N. E. Kuz’mina, V. M. Shchukin, E. Yu. Severinova, et al., Khim.-Farm. Zh., 49(7), 52 – 56 (2015); Pharm. Chem. J., 49(7), 490 – 494 (2015).
A. V. Skal’nyi and I. A. Rudakov, Bioelements in Medicine [in Russian], Mir, Moscow (2004).
N. E. Kuz’menko and S. S. Churanov, Obshch. Neorganich. Khim., Moscow State University, Moscow (1977).
Guideline on the specification limits for residues of metal catalysts or metal reagents, 2008 EMEA / CHMP / SWP / 4446 / 2000.
K. A. Fliszar, D. Walker, L. Allain, PDA J. Pharm. Sci. Technol., 60(6), 337 – 342 (2006).
Q3D. Guideline for elemental impurities ICH (2014).
D. R. Jenke, C. L. M. Stults, D. M. Paskiet, et al., J. Pharm. Sci. Technol., 69(1), 1 – 48 (2015).
S. V. Guzhova, N. N. Simonova, A. G. Liakumovich, et al., Vestnik Rozdravnadzora, No. 5, 44 – 49 (2013).
A. J. DeStefano, K. Zaidia, T. L. Cecil, and G. I. Giancaspro, Elemental impurities–Information. Pharmacopeial Forum, 36(1), 1 – 9 (2010).
I. V. Gravel’ and E. A. Plykina, Tradits. Med., 20(1), 49 – 54 (2010).
Unites States Pharmacopeia 39th edition. United States Pharmacopeial Convention; [Online resource], http: // www.uspnf.com / uspnf / .
European Pharmacopoeia, 9th ed. Council of Europe; [Online resource], http: // online6.edqm.eu / ep900 / .
Japanese Pharmacopoeia, 17th ed.; [Online resource], URL: http: // jpdb.nihs.go.jp / jp17e / .
Technical Guide for the Elaboration of Monographs, European Pharmacopoeia, European Directorate for the Quality of Medicines, 4 ed., 67 (2005).
Technical Guide for the Elaboration of Monographs, European Pharmacopoeia, European Directorate for the Quality of Medicines, 4 ed., 72 (2010).
K. B. Blake, Harmonization of the USP, EP, and JP heavy metals testing procedure. Pharmacopeial Forum, 21(6), 1632 – 1637 (1995).
V. Balaram, Trends Anal. Chem., 80, 83 – 95 (2016).
A. N. Novoselov, Vestnik VGU, ser. Khimiya. Biologiya. Farmatsiya, No. 2, (2012); [Online resource], http: // www.vestnik. vsu.ru / pdf / chembio / 2012 / 02 / 2012 – 02 – 10.pdf.
Unites States Pharmacopeia, 40th ed., United States Pharmacopeial Convention; [Online resource], http: // www.uspnf.com / uspnf / .
https: // www.edqm.eu / sites / default / files / press release pheur policy on elemental impurities update January 2017.pdf.
http: // www.usp.org / sites / default / files / usp / document / our-work / chemical-medicines / key-issues / 2009-04- 22 Metal ImpuritiesComment Digest.pdf.
European Pharmacopoeia, 9 ed. Supplement 9.3, Council of Europa; [Online resource], URL: http: // online6.edqm.eu / ep903 /.
USSR State Pharmacopeia, VII ed. [in Russian], Moscow (1937) p. 574.
USSR State Pharmacopeia, VIII ed. [in Russian], Moscow (1951) p. 632.
USSR State Pharmacopeia, IX ed. [in Russian], Moscow (1961) p. 676.
USSR State Pharmacopeia, X ed. [in Russian], Meditsina, Moscow (1968) p. 752.
USSR State Pharmacopeia, XI ed. [in Russian], Meditsina, Moscow (1998) p. 398.
Russian Federation State Pharmacopeia, XII ed. [in Russian], Yurgel’ N. V. (ed.), part 1, “Scientific Center for Expert Assessment of Medicines” Press, Moscow (2008).
Russian Federation State Pharmacopeia, XIII ed. [in Russian], Vol. 1; [Online resource]; URL: http: // 193.232.7.120 / feml / clinical ref / pharmacopoeia 1 / HTML /# (date accessed: 16.09.2016).
State Register of Medicines [in Russian]; [Online resource], http: // grls.rosminzdrav.ru / Default.aspx.
Russian Federation Government Order of October 23, 2017 No. 2323-r, List of Essential and Most Important Medicines for 2018, Moscow (2017).
I. V. Gravel’, I. P. Rudakova, and I. A. Samylina, Farmatsiya, 65(8), 9 – 13 (2016).
Author information
Authors and Affiliations
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 52, No. 1, pp. 54 – 59, January, 2018.
Rights and permissions
About this article
Cite this article
Kovaleva, E.L., Belanova, A.I., Panova, L.I. et al. Current Requirements for Assessment of Elemental Impurities (Heavy Metals) in Medicines. Pharm Chem J 52, 84–89 (2018). https://doi.org/10.1007/s11094-018-1769-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-018-1769-6