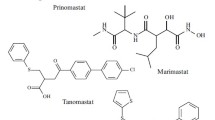
The new MMP-9 inhibitor 1-{4-[(4-chlorobenzoyl)amino]phenyl}sulfonyl-L-proline, with a theoretical inhibition constant of IC50 = 4 × 105 M, was constructed on the basis of structural requirements for selective inhibitors of gelatinases. This constructed compound and its close structural analogs were synthesized and these substances were found to have low toxicity, (LD50 > 300 mg/kg). The new inhibitor given p.o. at a dose of 20 mg/kg/day on the background of acute myocardial infarction significantly decreased the content of immunoreactive MMP-9 in plasma in rats, to the level obtained with doxycycline.






Similar content being viewed by others
References
P. Van Lint and C. Libert, J. Leukoc. Biol., 82, 1375 – 1381 (2007).
M. Hori and K. Nishida, Cardiovasc. Res., 81(3), 457 – 464 (2009).
F. G. Spinale, Physiol. Rev., 87, 1285 – 1342 (2007).
A. G. Gasanov and T. V. Bershova, Biomed. Khim., 55(2), 155 – 168 (2009).
K. S. Moshal, Physiol. Res., 57, 379 – 384 (2008).
J. Simova and J. J. Serum, Folia Biol., 59(5), 181 – 187 (2013).
P. Jain, C. Saravanan, and S. K. Singh, Eur. J. Med. Chem., 60, 89 – 100 (2013).
T. J. Peterson, H. Hallak, L. Johnson, et al., Circulation, 103, 2303 – 2309 (2001).
L. E. Rohde, A. Ducharme, L. H. Arroyo, et al., J. Am. Heart Assoc., 3063 – 3070 (1999).
W. M. Yarbrough, R. Mukherjee, G. P. Escobar, et al., Circulation, 108, 1753 – 1759 (2003).
M. P. Hudson, P. W. Armstrong, W. Ruzyllo, et al., J. Am. Coll. Cardiol., 48, 15 – 20 (2006).
R. P. Verma and S. Hansch, Bioorg. Med. Chem., 5, 2223 – 2268 (2007).
D. P. Becker, T. E. Barta, L. J. Bedell, et al., J. Med. Chem., 53, 6653 – 6680 (2010).
J. M. Cathcart and J. Cao, Frontiers in Bioscience, Landmark, 20, 1164 – 1178 (2015).
M. Whittaker, C. D. Floyd, P. Brown, et al., Chem. Rev., 99, 2735 – 2776 (1999).
G. Cerisano, P. Buonamici, A. M. Gori, et al., Int. J. Cardiol., 197, 147 – 153 (2015).
G. Cerisano, P. Buonamici, R. Valenti, et al., Eur. Heart J., 1 – 8 (2013).
G. Cerisano, P. Buonamici, R. Valenti, et al., Basic Res. Cardiol., 1 – 9 (2014).
T. Sadowski and J. Steinmeyer, Inflamm. Res., 50(3), 175 – 82 (2001).
M. L. Lindsey, Global J. Hum. Anat. Physiol. Res., 1, 6 – 9 (2014).
C. Camodeca, E. Nuti, L. Tepshi, et al., Eur. J. Med. Chem., 111, 193 – 201 (2016).
R. Oltenfreiter, L. Staelens, A. Lejeune, et al., Nucl. Med. Biol., 31, 459 – 468 (2004).
A. Tochowicz, K. Maskos, R. Huber, et al., J. Mol. Biol., 371, 989 – 1006 (2007).
M. Whittaker and A. Ayscough, Celltransmissions, 17(1), 3 – 14 (2001).
B. Pirad and H. Matter, J. Med. Chem., 49(1), 51 – 69 (2006).
US Patent No. 5985900 A (1999).
Y. Tamura, F. Watanabe, T. Nakatani, et al., J. Med. Chem., 41, 640 – 649 (1998).
D. Yamamoto and S. Takai, Cur. Med. Chem., 16, 1349 – 1354 (2009).
R. A. Friesner, R. B. Murphy, M. P. Repasky, et al., J. Med. Chem., 49, 6177 – 6196 (2006).
T. A. Halgren, R. B. Murphy, R. A. Friesner, et al., J. Med. Chem., 47, 1750 – 1759 (2004).
Schrodinger Release 2015-4: Maestro, version 10.4, Schrodinger, LLC, New York, NY (2015).
R. J. Cremlyn, F. S. Swinbourne, A. Batchelor, et al., Indian J. Chem., Section B: Organic Chem. Including Med. Chem., 2, No. 10, 1029 – 1043 (1983).
Y. Wang, D. Zhu, L. Tang, et al., Angew. Chem. Int. Ed., 50, 8917 (2011).
M. Tamura, D. Murase and K. Komura, Synthesis (Germany), 47(6), 769 – 776 (2015).
A. Van den Nieuwendijk, D. Pietra, L. Heitman, et al., J. Med. Chem., 47, 663 – 672 (2004).
J. DeRuiter, R. F. Borne, and C. A. Mayfield, J. Med. Chem., 32(1), 145 – 151 (1989).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 52, No. 1, pp. 8 – 14, January, 2018.
Rights and permissions
About this article
Cite this article
Grigorkevich, O.S., Mokrov, G.V., Dyabina, A.S. et al. Design, Synthesis, and Pharmacological Activity of a New Matrix Metalloproteinase-9 Inhibitor. Pharm Chem J 52, 30–36 (2018). https://doi.org/10.1007/s11094-018-1761-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-018-1761-1