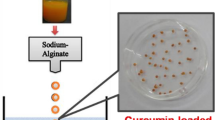
This study was aimed at the preparation of curcumin-loaded alginate/pectin microparticles (beads) in order to enhance the pharmaceutical effect of curcumin as a drug for colon related diseases. For this purpose, three types of curcumin-loaded beads were prepared with different alginate/pectin ratios (100/0, 75/25 and 50/50) and studied in various experiments including the release of curcumin from beads and swelling of beads in buffer solutions of simulated gastric, intestinal, and colon fluids. The results of curcumin releasing experiments under conditions mimicking stomach to colon transit showed that the beads with higher content of pectin exhibit stronger curcumin entrapment, slower release rate, and lower swelling. Thus, 50/50 alginate/pectin composition has minimum release in the upper parts of gastrointestinal tract (stomach and intestine). However, when these beads reached colonic buffer medium, the curcumin release suddenly increased. Therefore, beads with 50/50 alginate/pectin compositions could be useful as a suitable carrier for curcumin delivery to colon. Moreover, the stability and chemical protection of curcumin encapsulated in these beads was confirmed by high performance liquid chromatography measurements after a period of six months.




Similar content being viewed by others
References
S. Bisht, G. Feldmann, S. Soni, et al., J. Nanobiotechnol., 5(3), 1 – 18 (2007).
H. H. Tonnesen, M. Masson, and T. Loftsson, Int. J. Pharmaceutics, 244, 127 – 135 (2002).
J. J. Johnson and H. Mukhtar, Cancer Lett., 255, 170 – 181 (2007).
S. Shishodia, M. M. Chaturvedi and B. B. Aggarwal, Curr. Probl. Cancer, 31, 243 – 305 (2007).
S. Singh and A. Khar, Anticanc. Agents Med. Chem., 6, 259 – 270 (2006).
R. K. Maheshwari, A. K. Singh, J. Gaddipati and R. C. Srimal, Life Sci., 78, 2081 – 2087 (2006).
R. A. Sharma, A. J. Gescher and W. P. Steward, Eur. J. Cancer , 41, 1955 – 1968 (2005).
D. P. Chauhan, Curr. Pharm. Des., 8, 1695 – 1706 (2002).
S. C. Thomasset, D. P. Berry, G. Garcea, et al., Int. J. Cancer, 120, 451 – 458 (2007).
M. Lopez-Lazaro, Mol. Nutr. Food Res., 52, 103 – 127 (2008).
Y. Xu, C. Zhan, L. Fan, et al., Int. J. Pharm., 336, 329 – 337 (2007).
Y. J. Kim, H. G. Park, Y. L. Yang, et al., Biol. Pharm. Bull., 28(2), 394 – 397 (2005).
C. K. Siew and P. A. Williams, Biomacromolecules, 6, 963 – 969 (2005).
L.-S. Liu, M. L. Fishman, J. Kost and K. B. Hicks, Biomaterials, 24, 3333 – 3343 (2003).
Y. Fang, S. Al-Assaf, G. O. Phillips, et al., Carbohydr. Polym., 72, 334 – 341 (2008).
P. Sriamornsak, S. Sungthongjeen and S. Puttipipatkhachorn, Carbohydr. Polym., 67(3) 436 – 445 (2007).
P. Sriamornsak, Int. J. Pharm., 169, 213 – 220 (1998).
H. H. Tonnesen, Pharmazie, 61(8), 696 – 700 (2006).
P. Wakenstrom, S. Kidman, A.-M. Hermansson, et al., Food Hydrocoll., 17, 593 – 603 (2003).
V. Pillay and R. Fassihi, J. Control. Release, 59, 243 – 256 (1999).
L. Xing, C. Dawei, X. Liping and Z. Rongqing, J. Control. Release, 93, 293 – 300 (2003).
P. Y. Zhan, X. H. Zeng, XH, H. M. Zhang and H. H. Li, Food Chem., 129 (2), 700 – 703 (2011).
T. C. F. do Nascimento, D. M. Casa, L. F. Dalmolin, et al., Curr. Pharm. Anal., 8(4), 324 – 333 (2012).
M. J. Scotter, LWT Food Sci. Technol., 42, 1345 – 1351 (2009).
G. Liang, L. Shao, Y. Wang, et al., Bioorg. Medicinal Chem., 17, 2623 – 2631 (2009).
Y. J. Wang, M. H. Pan, A. L. Cheng, et al., Pharm. Biomed. Anal., 15, 1867 – 1876 (1997).
P.-H. Bong, Bull. Korean Chem. Soc., 21(1), 81 – 86 (2000).
Acknowledgements
We would like to thank the Research Councils of Shiraz University of Medical Sciences (6733), University of Tehran, and the Iran National Science Foundation (INSF) for supporting this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sattarahmady, N., Moosavi-Movahedi, A.A., Bazzi, P. et al. Improving Pharmaceutical Characteristics of Curcumin by Alginate/Pectin Microparticles. Pharm Chem J 50, 131–136 (2016). https://doi.org/10.1007/s11094-016-1410-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-016-1410-5