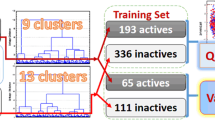
The relationship between molecular characteristics and hepatoprotector activity (increasing liver repair potential) was investigated for a diverse series of chemical compounds. Application of the nearest-neighbor method using geometric descriptors to a training set including 44 compounds (23 active and 21 inactive) gave combinations of the descriptors that classified correctly 79 – 91% of the training-set compounds. It was shown that a classifier that classified correctly all training-set compounds could be constructed by applying a simple voting procedure to results obtained for various combinations of descriptors. The effectiveness of the proposed approach for predicting the activity of untested compounds was proved by cross-validation with groups of compounds removed from the training set.
Similar content being viewed by others
References
L. A. Piruzyan, A. S. Kabankin, L. I. Gabrielyan, et al., Khim.-farm. Zh., 38(3), 19–25 (2004).
A. S. Kabankin, L. I. Gabrielyan, Khim.-farm. Zh., 39(3), 23–27 (2005).
A. S. Kabankin, L. I. Radkevich, L. I. Gabrielyan, et al., Khim.-farm. Zh., 39(4), 24–28 (2005).
A. S. Kabankin, L. I. Gabrielyan, Khim.-farm. Zh., 40(6), 17–21 (2006).
A. S. Kabankin, L. I. Gabrielyan, Khim.-farm. Zh., 42(5), 30–32 (2008).
L. A. Radkevich, Author’s Abstract of a Doctoral Dissertation in Biological Sciences, Moscow (1998).
R. Todeschini, V. Consonni, Handbook of Molecular Descriptors, Wiely-VCH, Weinheim, Germany (2000).
Milano Chemometrics & QSAR Research Group, DRAGON Web version 2.1 from http://michem.disat.unimib.it/chm/
P. Willett, J. M. Barnard, G. M. Downs, J. Chem. Inf. Comput. Sci., 38(6), 983–996 (1998).
D. K. Agrafiotis, W. Cedeno, V. S. Lobanov, J. Chem. Inf. Comput. Sci., 42(4), 903–911 (2002).
V. Svetnik, A. Liaw, C. Tong, et al., J. Chem. Inf. Comput. Sci., 43(6), 1947–1958 (2003).
C. Merkwirth, H. Mauser, T. Schulz-Gasch, et al., J. Chem. Inf. Model., 44(6), 1971–1978 (2004).
R. Guha, P. C. Jurs, J. Chem. Inf. Model., 44(6), 2179–2189 (2004).
H. Hong, W. Tong, Q. Hie, et al., SAR QSAR Environ. Res., 16(4), 339–347 (2005).
V. Svetnik, T.Wang, C. Tong, et al., J. Chem. Inf. Model., 45(3), 786–799 (2005).
T. Arodz, D. A. Yuen, A. Z. Dudek, J. Chem. Inf. Model., 46(1), 416–423 (2006).
Q. Zhang, J. M. Hughes-Oliver, R. T. Ng, J. Chem. Inf. Model., 49(8), 1857–1865 (2009).
P. M. Vasil’ev, Author’s Abstract of a Doctoral Dissertation in Biological Sciences, Volgograd (2009).
M. Culp, K. Johnson, G. Michalidis, J. Chem. Inf. Model., 50(2), 309–316 (2010).
I. J. Izenman, Modern Multivariate Statistical Techniques: Regression, Classification, and Manyfold Learning, Springer Science + Business Media, New York (2008), pp. 505–550.
T. Hastie, R. Tibshirani, J. Friedman, The Elements of Statistical Learning: Data Mining, Inference, and Prediction, Springer Science + Business Media, New York (2009).
A. B. Merkov, Sample Recognition: Introduction to Statistical Learning Methods [in Russian], Editorial URSS, Moscow (2011), pp. 183–205.
Author information
Authors and Affiliations
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 47, No. 4, pp. 3 – 8, April, 2013.
Rights and permissions
About this article
Cite this article
Kabankin, A.S., Radkevich, L.A. Collective recognition strategy for estimating hepatoprotector activity of various chemical compounds in increasing liver repair potential. Pharm Chem J 47, 181–186 (2013). https://doi.org/10.1007/s11094-013-0922-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-013-0922-5