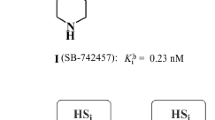
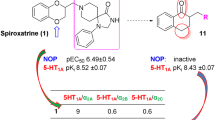
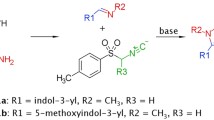
Both novel and previously reported (4-phenylsulfonyloxazol-5-yl)amine derivatives containing a substituted sulfonyl group were studied in order to discover highly active 5-HT6 receptor antagonists. A new pharmacophore model of 5-HT6 receptor antagonists was proposed based on the structure—5-HT6 antagonist activity relationship. It was established that the activity of the synthesized compounds depended strongly on the nature of the amine located vicinal to the sulfonyl. The most active ligands were methyl-(4-phenylsulfonyloxazol-5-yl)amines. Replacing the methyl by a bulkier alkyl radical and conversion to tertiary amines was accompanied by a dramatic reduction of their activity for 5-HT6 receptors.



Similar content being viewed by others
References
A. V. Ivachtchenko, Y. A. Ivanenkov, and S. E. Tkachenko, Expert Opin. Ther. Pat., 20(7), 1247 – 1257 (2010).
W. J. Geldenhuys and C. J. Van der Schyf, Expert Rev. Neurother., 9(7), 1073 – 1085 (2009).
D. J. Heal, S. L. Smith, A. Fisas, et al., Pharmacol. Ther., 117(2), 207 – 231 (2008).
A. V. Ivachtchenko, D. E. Dmitriev, E. S. Golovina, et al., J. Med. Chem., 53(14), 5186 – 5196 (2010).
F. Borsini (ed.), Pharmacology of 5-HT 6 receptors, Part I, Int. Rev. Neurobiol., Academic Press, (2010), p. 95.
M. L. Lopez-Rodriguez, B. Benhamu, T. de la Fuente, et al., J. Med. Chem., 48(13), 4216 – 4219 (2005).
A. V. Ivashchenko, E. S. Golovina, O. D. Mit?kin, et al., in: New Directions in the Chemistry of Heterocyclic Compounds [in Russian], Materials of the Second International Scientific Conference, Grafa, Stavropol? (2011), p. 159.
A. V. Ivachtchenko, E. S. Golovina, M. G. Kadieva, et al., Bioorg. Med. Chem. Lett., 22(13), 4273 – 4280 (2012).
K. G. Liu and A. J. Robichaud, Drug Dev. Res., 70, 145 – 168 (2009).
A. V. Ivachtchenko, D. E. Dmitriev, E. S. Golovina, et al., Bioorg. Med. Chem. Lett., 20(7), 2133 – 2136 (2010).
A. V. Ivachtchenko, E. S. Golovina, M. G. Kadieva, et al., Eur. J. Med. Chem., 46, 1189 – 1197 (2011).
A. V. Ivachtchenko, E. S. Golovina, M. G. Kadieva, et al., Bioorg. Med. Chem., 19(4), 1482 – 1491 (2011).
A. V. Ivashchenko, E. S. Golovina, and O. D. Mit?kin, in: Progress in Synthesis and Complexation [in Russian], Abstracts of Papers of the All-Russian Scientific Conference with International Participation Dedicated to the International Year of Chemistry, April 18 – 22, 2011, RUDN, Moscow (2011).
A. V. Ivachtchenko, E. S. Golovina, M. G. Kadieva, et al., J. Med. Chem., 54, 8161 – 8173 (2011).
A. V. Ivashchenko, E. S. Golovina, M. G. Kadieva, et al., Khim.-farm. Zh., 46(5), 48 – 58 (2012).
H. Greiner, G. Bartoszyk, H. Boettcher, et al., Pat. WO2002 / 100842.
R. Gericke, H. Bottcher, M. Gassen, and H. Greiner, Pat. WO2001 / 28316.
H. Greiner, G. Bartoszyk, H. Boettcher, et al., Pat. WO2000 / 037452.
V. A. Chervonnyi, A. V. Kharchenko, and B. S. Drach, Zh. Org. Khim., 34(2), 453 – 454 (1988).
http://www.electrochem.cn/~cheminfo/aids/moleculargraphics/DSViewerPro/DSVPMolecularOverlay.pdf
I. M. Okun, S. E. Tkachenko, A. Khvat, et al., Curr. Alzheimer Res., 7(2), 97 – 112 (2010).
P. Kasila and H. Harney, http://las.perkinelmer.com/Content/RelatedMaterials/Posters/PSTLANCEcAMPGasGaiCoupled-Receptors.pdf.
C. Reimer, E. Borroni, B. Levet-Trafit, et al., J. Med. Chem., 46(7), 1273 – 1276 (2003).
N. Upton, T. T. Chuang, A. J. Hunter, and D. J. Virley, Neurotherapeutics, 5(3), 458 – 469 (2008).
Y. Cheng and W. H. Prusoff, Biochem. Pharmacol., 22, 3099 – 3108 (1973).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 46, No. 11, pp. 7 – 14, November, 2012.
Rights and permissions
About this article
Cite this article
Ivachtchenko, A.V., Golovina, E.S., Kadieva, M.G. et al. 5-Ht6 Receptor Antagonists. V. Structure – Activity Relationship of (4-Phenylsulfonyloxazol-5-yl)amines. Pharm Chem J 46, 639–646 (2013). https://doi.org/10.1007/s11094-013-0861-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-013-0861-1