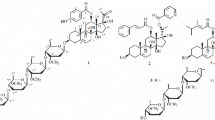
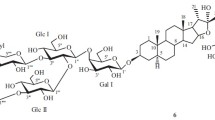
A plantation of Yucca gloriosa L. (mound-lily yucca) was created in eastern Georgia as a source of the sapogenin tigogenin and for raw material for the synthesis of steroidal hormone preparations of the 5α series. Leaves drying on the lower tier of the living plant contained only spirostanol glycosides. The dominant components of yuccaloesides A, B, and C were extracted from these, along with the new compound 3-O-α-L-rhamnopyranoside(1→4)-O-β-D-xylopyranosyl(1→3)-O-[β-D-glucopyranosyl(1→2)]-O-β-D-gluc opyranosyl(1→4)-O-β-D-galactopyranosyl 25R,5α-spirostan-3β-ol. Total glycosides from leaves drying on living plants were used to prepare a potential antimycotic substance for external application, Gloriofucin.
Similar content being viewed by others
References
É. P. Kemertelidze and T. A. Pkheidze, Khim.-Farm. Zh., 6, No. 12, 44–47 (1972).
N. I. Men’shova, N. N. Suvorov, É. P. Kemertelidze, et al., Khim.-Farm. Zh., 8, No. 7, 15–17 (1974).
E. Kemertelidze, G. Pkheidze, G. Grinenko, and N. Men’shova, Planta Med., 36, No. 3, 265–266 (1979).
L. K. Kavtaradze, R. I. Dabrundashvili, N. I. Men’shova, et al., Soobshch. AN GSSR, 132, No. 3, 537–539 (1988).
N. Sh. Nadaraya, M. D. Mashkovskii, É. P. Kemertelidze, et al., Khim.-Farm. Zh., 22, No. 3, 288–291 (1988).
M. I. Merlani, É. P. Kemertelidze, K. Papadopulos, and N. I. Men’shova, Bioorgan. Khim., 30, No. 5, 552–557 (2004).
M. I. Merlani, L. Sh. Amiranashvili, N. I. Men’shova, and É. P. Kemertelidze, Khim. Prirod. Soedin., 1, 81–82 (2007).
A. M. Dzhorbenadze and A. Ya. A. Ya. Shtromberg, Rastit. Res., 1, 97–103 (1972).
É. P. Kemertelidze, T. A. Pkheidze, L. N. Gvazava, et al., Khim. Prirod. Soedin., 2, 244–246 (1991).
M. M. Benidze, O. D. Dzhikiya, T. A. Pkheidze, et al., Khim. Prirod. Soedin., 4, 537–542 (1987).
M. M. Benidze, T. A. Pkheidze, and É. P. Kemertelidze, Khim. Prirod. Soedin., 2, 295–296 (1991).
É. Kemertelidze and M. Benidze, Bull. Georg. Acad. Sci., 164, No. 1, 91–93 (2001).
M. A. Laciaille-Dubois and H. Wagner, Phytomedicine, 2, 363–386 (1996).
Masazami Miyakoshi, Yukiyshi Tamura, Hitoshi Masuda, et al., J. Nat. Prod., 63, 332–338 (2000).
A. Favel, M. D. Steinmetz, P. Regli, et al., Planta Med., 60, 50–53 (1994).
A. Favel, É. Kemertelidze, M. Benidze, et al., Phytother. Res., 19, 158–161 (2005).
Author information
Authors and Affiliations
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 43, No. 1, pp. 27–29, January, 2009.
Rights and permissions
About this article
Cite this article
Kemertelidze, É.P., Benidze, M.M. & Skhirtladze, A.V. Steroid compounds from Yucca gloriosa L. introduced into Georgia and their applications. Pharm Chem J 43, 45–47 (2009). https://doi.org/10.1007/s11094-009-0230-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-009-0230-2