Abstract
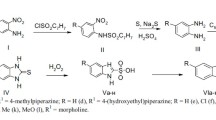
The synthesis and pharmacological properties of a new taurine amide derivative, N-(l-methyl-2-phenylethyl) aminoethanesulfonic acid isopropylamide hydrochloride (I), are described. Pronounced antiarrhythmic effect of compound I was observed in animals with experimental ventricular arrhythmias (early postocclusive and calcium chloride models). The drug significantly decreased the frequency and intensity of paroxysmal tachycardia attacks and ventricular fibrillation after coronary artery occlusion. Simultaneous administration of calcium chloride and compound I increased the arrhythmogenic dose of calcium chloride, inducing lethal heartbeat disorder and asystolia, and decreased the frequency of ventricular flutter and fibrillation. The antiarrhythmic effect of the drug was similar to that of lidocaine. The antifibrillatory activity is the most important manifestation of the antiarrhythmic action of the taurine derivative studied. These data suggest that compound I is a potential drug for urgent aid in myocardial infarction.
Similar content being viewed by others
References
I. I. Knyaz’kova, I. A. Tsygankov, and Yu. G. Gorb, Current Problems in Therapy (A Coll. of Sci. Papers) [in Russian], Kharkov (1992), pp. 121–131.
R. J. Huxtable, Physiol. Rev, 72, 101–163 (1992).
N. S. Sapronov and L. K. Gavrovskaya, Adv. Exp. Med. Boil., 286, 509–513 (2006).
L. I. Nefedov, Vest. Akad. Nav. Belarusi, Ser. Biyal. Navuk, No. 3–4, 99–107 (1992).
H. Selye, E. Bayusz, S. Grasso, et al., Angiology, 11, 398–407 (1960).
M. J. Walker, M. J. Curtis, D. J. Hearse, et al., Cardiovasc. Res, 22, 447–455 (1988).
RF Patent No. 2185159; Byul. Izobret., No. 20 (2002).
Author information
Authors and Affiliations
Additional information
__________
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 41, No. 11, pp. 19–21, November, 2007.
Rights and permissions
About this article
Cite this article
Sapronov, N.S., Gavrovskaya, L.K., Krylova, I.B. et al. Synthesis and antiarrhythmic activity of n-(1-methyl-2-phenylethyl)aminoethanesulfonic acid isopropylamide hydrochloride. Pharm Chem J 41, 585–587 (2007). https://doi.org/10.1007/s11094-008-0027-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-008-0027-8