Abstract
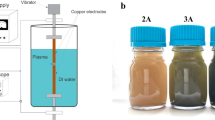
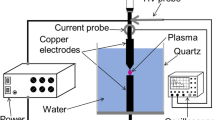
The influence of the liquid composition on the chemical and morphological properties of copper-based nanostructures synthesized by a non-equilibrium atmospheric plasma treatment is investigated and discussed. The synthesis approach is simple and environmentally friendly, employs a non-equilibrium nanopulsed atmospheric pressure plasma jet as a contactless cathode and a Cu foil as immersed anode. The process was studied using four distinct electrolyte solutions composed of distilled water and either NaCl + NaOH, NaCl only or NaOH only at two different concentrations, without the addition of any copper salts. CuO crystalline structures with limited impurities (e.g. Cu and Cu(OH)2 phases) were produced from NaCl + NaOH containing solutions, mainly CuO and CuCl2 structures were synthesized in the electrolyte solution containing only NaCl and no synthesis occurred in solutions containing only NaOH. Both aggregated and dispersed nanostructures were produced in the NaCl + NaOH and NaCl containing solutions. Reaction pathways leading to the formation of the nanostructures are proposed and discussed.






Similar content being viewed by others
References
Graves DB (2012) The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J Phys D Appl Phys 45:263001. https://doi.org/10.1088/0022-3727/45/26/263001
Bruggeman P, Leys C (2009) Non-thermal plasmas in and in contact with liquids. J Phys D Appl Phys 42:053001. https://doi.org/10.1088/0022-3727/42/5/053001
Wei-Hung C, Carolyn R, Sankaran RM (2010) Continuous-flow, atmospheric-pressure microplasmas: a versatile source for metal nanoparticle synthesis in the gas or liquid phase. Plasma Sources Sci Technol 19:34011. https://doi.org/10.1088/0963-0252/19/3/034011
Patel J, Němcová L, Maguire P et al (2013) Synthesis of surfactant-free electrostatically stabilized gold nanoparticles by plasma-induced liquid chemistry. Nanotechnology 24:245604. https://doi.org/10.1088/0957-4484/24/24/245604
Du C, Xiao M (2014) Cu2O nanoparticles synthesis by microplasma. Sci Rep 4:7339. https://doi.org/10.1038/srep07339
Mariotti D, Patel J, Svrcek V, Maguire P (2012) Plasma–liquid interactions at atmospheric pressure for nanomaterials synthesis and surface engineering. Plasma Process Polym 9:1074–1085. https://doi.org/10.1002/ppap.201200007
Rumbach P, Bartels DM, Sankaran RM, Go DB (2015) The effect of air on solvated electron chemistry at a plasma/liquid interface. J Phys D Appl Phys 48:424001. https://doi.org/10.1088/0022-3727/48/42/424001
Rumbach P, Witzke M, Sankaran RM, Go DB (2013) Decoupling interfacial reactions between plasmas and liquids: charge transfer vs plasma neutral reactions. J Am Chem Soc 135:16264–16267. https://doi.org/10.1021/ja407149y
Rumbach P, Griggs N, Sankaran RM, Go DB (2014) Visualization of electrolytic reactions at a plasma–liquid interface. IEEE Trans Plasma Sci 42:2610–2611. https://doi.org/10.1109/TPS.2014.2322976
Mariotti D, Sankaran RM (2010) Microplasmas for nanomaterials synthesis. J Phys D Appl Phys 43:323001. https://doi.org/10.1088/0022-3727/43/32/323001
Anžlovar A, Marinšek M, Orel ZC, Žigon M (2015) Basic zinc carbonate as a precursor in the solvothermal synthesis of nano-zinc oxide. Mater Des 86:347–353. https://doi.org/10.1016/j.matdes.2015.07.087
Yu-Ping Z, Lee S-H, Reddy KR et al (2007) Synthesis and characterization of core–shell SiO2 nanoparticles/poly (3-aminophenylboronic acid) composites. J Appl Polym Sci 104:2743–2750. https://doi.org/10.1002/app
Hassan M, Haque E, Reddy KR et al (2014) Edge-enriched graphene quantum dots for enhanced photo-luminescence and supercapacitance. Nanoscale 6:11988–11994. https://doi.org/10.1039/C4NR02365J
Tan C, Zhang H (2015) Wet-chemical synthesis and applications of non-layer structured two-dimensional nanomaterials. Nat Commun 6:7873. https://doi.org/10.1038/ncomms8873
Reetz MT, Helbig W (1994) Size-selective synthesis of nanostructured transition metal clusters. J Am Chem Soc 116:7401–7402. https://doi.org/10.1021/ja00095a051
Richmonds C, Sankaran RM (2008) Plasma–liquid electrochemistry: rapid synthesis of colloidal metal nanoparticles by microplasma reduction of aqueous cations. Appl Phys Lett 93:2013–2016. https://doi.org/10.1063/1.2988283
Furuya K, Hirowatari Y, Ishioka T, Harata A (2007) Protective agent-free preparation of gold nanoplates and nanorods in aqueous HAuCl4 solutions using gas–liquid interface discharge. Chem Lett 36:1088–1089. https://doi.org/10.1246/cl.2007.1088
Furusho H, Kitano K, Hamaguchi S, Nagasaki Y (2009) Preparation of stable water-dispersible PEGylated gold nanoparticles assisted by nonequilibrium atmospheric-pressure plasma jets. Chem Mater 21:3526–3535. https://doi.org/10.1021/cm803290b
Hieda J, Saito N, Takai O (2008) Exotic shapes of gold nanoparticles synthesized using plasma in aqueous solution. J Vac Sci Technol A Vac Surf Film 26:854. https://doi.org/10.1116/1.2919139
Jana NR, Gearheart L, Murphy CJ (2001) Wet chemical synthesis of high aspect ratio cylindrical gold nanorods. J Phys Chem B 105:19. https://doi.org/10.1021/jp0107964
Rogach AL (2000) Nanocrystalline CdTe and CdTe(S) particles: wet chemical preparation, size-dependent optical properties and perspectives of optoelectronic applications. Mater Sci Eng B Solid-State Mater Adv Technol 69:435–440. https://doi.org/10.1016/S0921-5107(99)00231-7
Dierstein A, Natter H, Meyer F et al (2001) Electrochemical deposition under oxidizing conditions (EDOC): a new synthesis for nanocrystalline metal oxides. Scr Mater 44:2209–2212. https://doi.org/10.1016/S1359-6462(01)00906-X
Chang S-S, Lee C-L, Wang CRC (1997) Gold nanorods: electrochemical synthesis and optical properties. J Phys Chem B 101:6661–6664. https://doi.org/10.1021/jp971656q
Wijesundera RP (2010) Fabrication of the CuO/Cu2O heterojunction using an electrodeposition technique for solar cell applications. Semicond Sci Technol 25:045015. https://doi.org/10.1088/0268-1242/25/4/045015
Anandan S, Wen X, Yang S (2005) Room temperature growth of CuO nanorod arrays on copper and their application as a cathode in dye-sensitized solar cells. Mater Chem Phys 93:35–40. https://doi.org/10.1016/j.matchemphys.2005.02.002
Sun H, Harms K, Sundermeyer J et al (2004) Aerobic oxidation of 2,3,6-trimethylphenol to trimethyl-1,4-benzoquinone with copper(II) chloride as catalyst in ionic liquid and structure of the active species. J Am Chem Soc 126:9550–9551
Rubilar O, Rai M, Tortella G et al (2013) Biogenic nanoparticles: copper, copper oxides, copper sulphides, complex copper nanostructures and their applications. Biotechnol Lett 35:1365–1375
Boselli M, Colombo V, Ghedini E et al (2014) Schlieren high-speed imaging of a nanosecond pulsed atmospheric pressure non-equilibrium plasma jet. Plasma Chem Plasma Process 34:853–869. https://doi.org/10.1007/s11090-014-9537-1
Colombo V, Fabiani D, Focarete ML et al (2014) Atmospheric pressure non-equilibrium plasma treatment to improve the electrospinnability of poly(L-Lactic Acid) polymeric solution. Plasma Process Polym 11:247–255. https://doi.org/10.1002/ppap.201300141
Liguori A, Pollicino A, Stancampiano A et al (2016) Deposition of plasma-polymerized polyacrylic acid coatings by a non-equilibrium atmospheric pressure nanopulsed plasma jet. Plasma Process Polym 13:375–386. https://doi.org/10.1002/ppap.201500080
Liguori A, Traldi E, Toccaceli E et al (2015) Co-deposition of plasma-polymerized polyacrylic acid and silver nanoparticles for the production of nanocomposite coatings using a non-equilibrium atmospheric pressure plasma jet. Plasma Process Polym. https://doi.org/10.1002/ppap.201500143
Laurita R, Barbieri D, Gherardi M et al (2015) Chemical analysis of reactive species and antimicrobial activity of water treated by nanosecond pulsed DBD air plasma. Clin Plasma Med 3:53–61. https://doi.org/10.1016/j.cpme.2015.10.001
Faita G, Fiori G, Salvadore D (1975) Copper behaviour in acid and alkaline brines—I kinetics of anodic dissolution in 0.5 M NaCl and free-corrosion rates in the presence of oxygen. Corros Sci 15:383–392
Yuan B, Wang C, Li L, Chen S (2009) Real time observation of the anodic dissolution of copper in NaCl solution with the digital holography. Electrochem Commun 11:1373–1376
Kear G, Barker BD, Walsh FC (2004) Electrochemical corrosion of unalloyed copper in chloride media—a critical review. Corros Sci 46:109–135
Ethiraj AS, Kang DJ (2012) Synthesis and characterization of CuO nanowires by a simple wet chemical method. Nanoscale Res Lett 7:70. https://doi.org/10.1186/1556-276X-7-70
Balamurugan B, Mehta BR (2001) Optical and structural properties of nanocrystalline copper oxide thin films prepared by activated reactive evaporation. Thin Solid Films 396:90–96. https://doi.org/10.1016/S0040-6090(01)01216-0
Sun S, Zhang X, Sun Y et al (2013) Facile water-assisted synthesis of cupric oxide nanourchins and their application as nonenzymatic glucose biosensor. ACS Appl Mater Interfaces 5:4429–4437. https://doi.org/10.1021/am400858j
Borgohain K, Singh J, Rama Rao M et al (2000) Quantum size effects in CuO nanoparticles. Phys Rev B 61:11093–11096. https://doi.org/10.1103/PhysRevB.61.11093
Vasquez RP, Foote MC, Hunt BD (1989) Reaction of nonaqueous halogen solutions with YBa2Cu3O7−x. J Appl Phys 66:4866–4877. https://doi.org/10.1063/1.343805
Krylova V, Andrulevicius M (2009) Optical, XPS and XRD studies of semiconducting copper sulfide layers on a polyamide film. Int J Photoenergy. https://doi.org/10.1155/2009/304308
Drogowska M, Brossard L, Menard H (1987) Anodic copper dissolution in the presence of Cl-ions at pH 12. Corrosion 43:549–552. https://doi.org/10.5006/1.3583899
Engelbrekt C, Malcho P, Andersen J et al (2014) Selective synthesis of clinoatacamite Cu2(OH)3Cl and tenorite CuO nanoparticles by pH control. J Nanopart Res 16:2561. https://doi.org/10.1007/s11051-014-2562-4
Wang W, Liu Z, Liu Y et al (2003) A simple wet-chemical synthesis and characterization of CuO nanorods. Appl Phys A Mater Sci Process 76:417–420. https://doi.org/10.1007/s00339-002-1514-5
Cudennec Y, Lecerf A (2003) The transformation of Cu(OH)2 into CuO, revisited. Solid State Sci 5:1471–1474. https://doi.org/10.1016/j.solidstatesciences.2003.09.009
Velusamy T, Liguori A, Macias-Montero M et al (2017) Ultra-small CuO nanoparticles with tailored energy-band diagram synthesized by a hybrid plasma–liquid process. Press Plasma Process Polym. https://doi.org/10.1002/ppap.201100001
Guo TX, Zhao Y, Ma SC, Liu ST (2012) Decomposition characteristics of hydrogen peroxide in sodium hydroxide solution. Adv Mater Res 610:359–362. https://doi.org/10.4028/www.scientific.net/AMR.610-613.359
Richardson HW (1997) Handbook of copper compounds and applications. CRC Press
Witzke M, Rumbach P, Go DB, Sankaran RM (2012) Evidence for the electrolysis of water by atmospheric-pressure plasmas formed at the surface of aqueous solutions. J Phys D Appl Phys 45:442001. https://doi.org/10.1088/0022-3727/45/44/442001
Acknowledgements
This work was partially supported by the PLASMAT Project (Alma Mater Studiorum—Università di Bologna, FARB Grant) and by COST Action TD1208 Electrical Discharges with Liquids for Future Applications. The authors would like to acknowledge Prof. Vittorio Colombo, Prof. Maria Letizia Focarete and Prof. Catia Arbizzani for the fruitful scientific conversations. This work was also partially supported by EPSRC (Award n.EP/M024938/1 and n.EP/K022237/1).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Liguori, A., Gallingani, T., Padmanaban, D.B. et al. Synthesis of Copper-Based Nanostructures in Liquid Environments by Means of a Non-equilibrium Atmospheric Pressure Nanopulsed Plasma Jet. Plasma Chem Plasma Process 38, 1209–1222 (2018). https://doi.org/10.1007/s11090-018-9924-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-018-9924-0