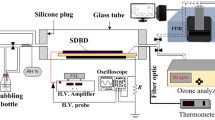
Abstract
The decomposition of naphthalene in surface dielectric barrier discharge (SDBD) based reactor is investigated in different carrier gases (air, nitrogen, and oxygen) in order to understand the reaction mechanism of the decomposition process. The decomposition efficiency of naphthalene is determined at different oxygen content (from 0 to 10 vol%) and different input power. The highest decomposition efficiency is obtained in nitrogen at low input power, due to the role played by the nitrogen excited species in the decomposition process. In addition, the decomposition efficiency is decreased with increasing the oxygen content at a low input power. At a relatively high input power, the decomposition efficiency reached its maximum value in pure oxygen. Moreover, the decomposition efficiency decreases with the increase of the oxygen content reaching minimum value at a small content of oxygen (~3 vol% O2) and relatively high input power, and then increases at higher oxygen content. The results show that the decomposition of naphthalene in the present reactor could be treated as a first order reaction with respect to the concentration of naphthalene.













Similar content being viewed by others
References
Harvey RG (1997) Polycyclic aromatic hydrocarbons. Wiley-VCH, New York
Petrov A (1987) Petroleum hydrocarbons. Springer, New York, NY
Atkinson R, Aschman S (1988) Kinetics of reactions of acenaphthene and acenaphthylene and structurally related aromatic compounds with OH and NO3 radicals, N2O5 and O3 at 296 K. Int J Chem Kinet 20:513–553
Szepesy L, Lakszner K, Ackermann L, Podmaniczky L, Literáthy P (1981) Rapid method for the determination of polycyclic aromatic hydrocarbons in environment samples by combined liquid and gas chromatography. J Chromatogr A 206:611–616
Ravindra K, Sokhi R, Vangrieken R (2008) Atmospheric polycyclic aromatic hydrocarbons: source attribution, emission factors and regulation. Atmos Environ 42:2895–2921
Beegle LW, Wdowiak TJ, Harrison JG (2001) Hydrogenation of polycyclic aromatic hydrocarbons as a factor affecting the cosmic 6.2 micron emission band. Spectrochim Acta Part A Mol Biomol Spectrosc 57:737–744
Marchand N, Besombes JL, Chevron N, Masclet P, Aymoz G, Jaffrezo JL (2004) Polycyclic aromatic hydrocarbons (PAHs) in the atmospheres of two French alpine valleys: sources and temporal patterns. Atmos Chem Phys 4:1167–1181
Keith L, Telliard W (1979) ES&T Special Report: priority pollutants: I—a perspective view. Environ Sci Technol 13:416–423
Bunce N, Liu L, Zhu J (1997) Reaction of naphthalene and its derivatives with hydroxyl radicals in the gas phase. Environ Sci Technol 31:2252–2259
Dann T (1989) Polycyclic aromatic hydrocarbons in the ambient air of Toronto, Ontario and Montréal, Québec. Pollution Measurement Division, Technology Development and Technology Services Branch, Environment Canada
Wang L, Atkinson R, Arey J (2007) Dicarbonyl products of the OH radical-initiated reactions of naphthalene and the C1- and C2-alkylnaphthalenes. Environ Sci Technol 41:2803–2810
Bityurin VA, Filimonova EA, Naidis GV (2009) Simulation of naphthalene conversion in biogas initiated by pulsed corona discharges. IEEE Trans Plasma Sci 37(6):911–919
Nair SA, Pemen AJM, Yan K, van Gompel FM, van Leuken HEM, van Heesch EJM, Ptasinski KJ, Drinkenburg AAH (2003) Tar removal from biomass-derived fuel gas by pulsed corona discharges. Fuel Process Technol 84:161–173
Ostapczuk A, Hakoda T, Shimada A, Kojima T (2008) Naphthalene and acenaphthene decomposition by electron beam generated plasma application. Plasma Chem Plasma Process 28:483–494
Yu L, Li X, Tu X, Wang Y, Lu S, Yan J (2010) Decomposition of naphthalene by dc gliding arc gas discharge. J Phys Chem A 114:360–368
Ni MJ, Shen X, Gao X, Wu Z, Lu H, Li Z, Luo Z, Cen KF (2011) Naphthalene decomposition in a DC corona radical shower discharge. J Zhejiang Univ Sci A 12:71–77
Liang W-J, Fang H-P, Li J, Zheng F, Li J-X, Jin Y-Q (2011) Performance of non-thermal DBD plasma reactor during the removal of hydrogen sulfide. J Electrostat 69:206–213
Byeon JH, Park JH, Jo YS, Yoon KY, Hwang J (2010) Removal of gaseous toluene and submicron aerosol particles using a dielectric barrier discharge reactor. J Hazard Mater 175:417–422
Mok YS, Lee S, Chang MS (2009) Destruction of chlorodifluoromethane (CHF2Cl) by using dielectric barrier discharge plasma. IEEE Trans Ind Appl 37:449–455
Redolfi M, Aggadi N, Duten X, Touchard S, Pasquiers S, Hassouni K (2009) Oxidation of acetylene in atmospheric pressure pulsed corona discharge cell working in the nanosecond regime. Plasma Chem Plasma Process 29:173–195
Lu B, Ji M, Wang MY, Lv J (2009) Ethanethiol removal using a novel plasma reactor combined with Mn/g–Al2O3. In: International conference on environmental science and information application technology, Wuhan, pp 623–626
Chen HL, Lee HM, Chen SH, Chang MB, Yu SJ, Li SN (2009) Removal of volatile organic compounds by single-stage and two-stage plasma catalysis systems: a review of the performance enhancement mechanisms, current status, and suitable applications. Environ Sci Technol 43:2216–2227
Zhu T, Li J, Jin Y, Liang Y, Ma G (2008) Decomposition of benzene by non-thermal plasma processing: photocatalyst and ozone effect. Int J Environ Sci Technol 5:375–384
Blin-Simiand N, Pasquiers S, Jorand F, Postel C, Vacher JR (2009) Removal of formaldehyde in nitrogen and in dry air by a DBD: importance of temperature and role of nitrogen metastable states. J Phys D Appl Phys 42:122003
Lu B, Ji M, Wang M, Lü J (2007) Plasma oxidation of benzene using DBD corona discharges. J Mater Eng Perform 17:428–431
Chang C, Bai H, Lu S (2005) Destruction of styrene in an air stream by packed dielectric barrier discharge reactors. Plasma Chem Plasma Process 25:641–657
Agnihotri S, Cal MP, Prien J (2004) Destruction of 1,1,1-trichloroethane using dielectric barrier discharge nonthermal plasma. J Environ Eng 130:349–355
Lee HM, Chang MB (2003) Abatement of gas-phase p-xylene via dielectric barrier discharges. Plasma Chem Plasma Process 23:541–558
Oda T, Takahashi T, Kohzuma S (2001) Decomposition of dilute trichloroethylene by using nonthermal plasma processing-frequency and catalyst effects. IEEE Trans Ind Appl 37:965–970
Chen J, Su Q, Pan H, Wei J, Zhang X, Shi Y (2009) Influence of balance gas mixture on decomposition of dimethyl sulfide in a wire-cylinder pulse corona reactor. Chemosphere 75:261–265
Guo Y, Ye D, Chen K, He J (2007) Toluene removal by a DBD-type plasma combined with metal oxides catalysts supported by nickel foam. Catal Today 126:328–337
Mok YS, Demidyuk V, Whitehead JC (2008) Decomposition of hydrofluorocarbons in a dielectric-packed plasma reactor. J Phys Chem A 112:6586–6591
Blin-Simiand N, Jorand F, Magne L, Pasquiers S, Postel C, Vacher JR (2008) Plasma reactivity and plasma-surface interactions during treatment of toluene by a dielectric barrier discharge. Plasma Chem Plasma Process 28:429–466
Falkenstein Z (1999) Effects of the O2 concentration on the removal efficiency of volatile organic compounds with dielectric barrier discharges in Ar and N2. J Appl Phys 85:525
Huang L, Nakajo K, Ozawa S, Matsuda H (2001) Decomposition of Dichloromethane in a wire-in-tube pulsed corona reactor. Environ Sci Technol 35:1276–1281
Abdelaziz A, Seto T, Abdel-Salam M, Otani Y (2012) Performance of a surface dielectric barrier discharge based reactor for destruction of naphthalene in an air stream. J Phys D Appl Phys 45:115201
Kogelschatz U, Eliasson B, Egli W (1997) Dielectric-barrier discharges: principle and applications. Le J Phys IV 07:C4-47-C44-66
Enloe CL, McHarg MG, McLaughlin TE (2008) Time-correlated force production measurements of the dielectric barrier discharge plasma aerodynamic actuator. J Appl Phys 103:073302
Kim H-H (2004) Nonthermal plasma processing for air-pollution control: a historical review, current issues, and future prospects. Plasma Processes Polym 1:91–110
Itikawa Y (2006) Cross sections for electron collisions with nitrogen molecules. J Phys Chem Ref Data 35:31–53
Herron J (2001) Modeling studies of the formation and destruction of no in pulsed barrier discharges in nitrogen and air. Plasma Chem Plasma Process 21:581–609
Itikawa Y (2009) Cross sections for electron collisions with oxygen molecules. J Phys Chem Ref Data 38:1–20
Acknowledgments
The authors would like to present deep thanks to Dr. T. Ishijima, Associate professor at Research Center for Sustainable Energy and Technology—Kanazawa University, for his help and suggestions in the optical spectroscopic measurements. Moreover, the authors express sincere appreciation to Walid M. Hikal, Postdoc in Texas Tech., for his valuable comments in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdelaziz, A.A., Seto, T., Abdel-Salam, M. et al. Influence of N2/O2 Mixtures on Decomposition of Naphthalene in Surface Dielectric Barrier Discharge Based Reactor. Plasma Chem Plasma Process 34, 1371–1385 (2014). https://doi.org/10.1007/s11090-014-9578-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-014-9578-5