Abstract
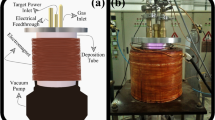
Nanostructured ZnO thin films have been deposited on glass substrate at low substrate temperature (200 °C) using the Spray Plasma technique. The zinc nitrate or zinc chloride precursors (droplets) are injected in a low-pressure Ar/O2 radio-frequency inductive discharge. The O2 fraction and the distance between the inductive coil and the substrate holder were varied, and the resulting effect on the deposited film properties was investigated by means of X-ray diffraction, atomic force microscopy, transmittance electron microscopy and UV–visible spectroscopy. It was shown that the c-axis orientation growth depends on these two parameters but not on the precursor nature. The quality of the deposited film is optimal for 5 % O2 in argon when the inductive coil-substrate distance is small. The Ar/O2 plasma was also diagnosed by optical emission spectroscopy. The oxygen atom relative concentration was monitored by optical emission actinometry and the rotational spectrum of OH was recorded to estimate the gas temperature.













Similar content being viewed by others
References
Repins I, Contreras MA, Egaas B, Dehart C, Scharf J, Perkins CL, Tofhsjh B, Noufi R (2008) 19.9%-efficient ZnO/CdS/CuInGaSe2 solar cell with 81.2% fill factor. Prog Photovolt 16(3):235–239
Ohta H, Kawamura K, Orita M, Hirano M, Sarukura N, Hosono H (2000) Current injection emission from a transparent p-n junction composed of p-SrCu2O2/n-ZnO. Appl Phys Lett 77(4):475–477
Mitra P, Chatterjee AP, Maiti HS (1998) ZnO thin film sensor. Mater Lett 35(1–2):33–38
Ootsuka T, Liu Z, Osamura M, Fukuzawa Y, Kuroda R, Suzuki Y, Otogawa N, Mise T, Wang S, Hoshino Y, Nakayama Y, Tanoue H, Makita Y (2005) Studies on aluminum-doped ZnO films for transparent electrode and antireflection coating of β-FeSi2 optoelectronic devices. Thin Solid Films 476(1):30–34
Minami T (2005) Transparent conducting oxide semiconductors for transparent electrodes. Semicond Sci Technol 20(4):S35–S44
Kaidashev EM, Lorenz M, von Wenckstern H, Rahm A, Semmelhack HC, Han KH, Benndorf G, Bundesmann C, Hochmuth H, Grundmann M (2003) High electron mobility of epitaxial ZnO thin films on c-plane sapphire grown by multistep pulsed-laser deposition. Appl Phys Lett 82(22):3901–3903
Wenas WW, Yamada A, Takahashi K, Yoshino M, Konagai M (1991) Electrical and optical-properties of boron-doped ZnO thin-films for solar-cells grown by metalorganic chemical vapor-deposition. J Appl Phys 70(11):7119–7123
Studenikin SA, Golego N, Cocivera M (1998) Fabrication of green and orange photoluminescent, undoped ZnO films using spray pyrolysis. J Appl Phys 84(4):2287–2294
Labeau M, Rey P, Deschanvres JL, Joubert JC, Delabouglise G (1992) Thin-films of high-resistivity zinc-oxide produced by a modified CVD method. Thin Solid Films 213(1):94–98
Walters G, Parkin IP (2009) Aerosol assisted chemical vapour deposition of ZnO films on glass with noble metal and p-type dopants; use of dopants to influence preferred orientation. Appl Surf Sci 255(13–14):6555–6560
Garnier J, Bouteville A, Hamilton J, Pemble ME, Povey IM (2009) A comparison of different spray chemical vapour deposition methods for the production of undoped ZnO thin films. Thin Solid Films 518(4):1129–1135
Park JH, Jang SJ, Kim SS, Lee BT (2006) Growth and characterization of single crystal ZnO thin films using inductively coupled plasma metal organic chemical vapor deposition. Appl Phys Lett 89(12):121108
Volintiru I, Creatore M, Kniknie BJ, Spee C, van de Sanden MCM (2007) Evolution of the electrical and structural properties during the growth of Al doped ZnO films by remote plasma-enhanced metalorganic chemical vapor deposition. J Appl Phys 102(4):043709
Li BS, Liu YC, Shen DZ, Lu YM, Zhang JY, Kong XG, Fan XW, Zhi ZZ (2002) Growth of high quality ZnO thin films at low temperature on Si(100) substrates by plasma enhanced chemical vapor deposition. J Vac Sci Technol A-Vac Surf Films 20(1):265–269
Li BS, Liu YC, Shen DZ, Zhang JY, Lu YM, Fan XW (2003) Effects of RF power on properties of ZnO thin films grown on Si (001) substrate by plasma enhanced chemical vapor deposition. J Cryst Growth 249(1–2):179–185
Baba K, Lazzaroni C, Brinza O, Nikravech M (2014) Effect of zinc nitrate concentration on structural and optical properties of ZnO thin films deposited by spray plasma device. Thin Solid Films 558:62–66
Baba K, Nikravech M, Vrel D, Kanaev A, Museur L, Chehimi M (2012) Characteristics of nanostructured ZnO layers deposited in spray plasma device. J Nanosci Nanotechnol 12(6):4744–4748
Nikravech M (2010) Spray plasma device, a new method to process nanostructured layers. application to deposit ZnO thin layers. J Nanosci Nanotechnol 10(2):1171–1178
Huber KP, Herzberg G (1979) Molecular spectra and molecular structure I, Chap V. Van Nostrand Reinhold, New York
Tonnis EJ, Graves DB (2002) Neutral gas temperatures measured within a high-density, inductively coupled plasma abatement device. J Vac Sci Technol A-Vac Surf Films 20(5):1787–1795
Garg RK, Anderson TN, Lucht RP, Fisher TS, Gore JP (2008) Gas temperature measurements in a microwave plasma by optical emission spectroscopy under single-wall carbon nanotube growth conditions. J Phys D-Appl Phys 41(9):95206–95214
Dieke GH, Crosswhite HM (1962) The ultraviolet bands of OH fundamental data. J Quant Spectrosc Radiat Transf 2(2):97–199
Pichamut Jp, Hassler JC, Coleman PD (1972) Gas-temperature measurement in pulsed H2O laser discharges. J Appl Phys 43(11):4562–4565
Sokabe N (1972) Rotational Excitation of OH (A2Σ+, v’ = 0) resulting from dissociative collision of H2O with metastable argon atoms. J Phys Soc Jpn 33(2):473
Lazzaroni C, Baba K, Nikravech M, Chabert P (2012) Model of a low-pressure radio-frequency inductive discharge in Ar/O2 used for plasma spray deposition. J Phys D Appl Phys 45:485207–485215
Williamson GK, Hall WH (1953) X-ray line broadening from filed aluminium and wolfram. Acta Metall 1(1):22–31
Lotgering FK (1959) Topotactical reactions with ferrimagnetic oxides having hexagonal crystal structures—I. J Inorg Nucl Chem 9(2):113–123
Huang YQ, Liu MD, Li Z, Zeng YK, Liu SB (2003) Raman spectroscopy study of ZnO-based ceramic films fabricated by novel sol-gel process. Mater Sci Eng B-Solid State Mater Adv Technol 97(2):111–116
Zhaochun Z, Baibiao H, Yongqin Y, Deliang C (2001) Mat Sci Eng B86:109–112
Wang C, Chen Z, Hu H, Zhang D (2009) Effect of the oxygen pressure on the microstructure and optical properties of ZnO films prepared by laser molecular beam epitaxy. Physica B 404(21):4075–4082
Ma Y, Du GT, Yang SR, Li ZT, Zhao BJ, Yang XT, Yang TP, Zhang YT, Liu DL (2004) Control of conductivity type in undoped ZnO thin films grown by metalorganic vapor phase epitaxy. J Appl Phys 95(11):6268–6272
Choopun S, Vispute RD, Noch W, Balsamo A, Sharma RP, Venkatesan T, Iliadis A, Look DC (1999) Oxygen pressure-tuned epitaxy and optoelectronic properties of laser-deposited ZnO films on sapphire. Appl Phys Lett 75(25):3947–3949
Nam KH, Kim H, Lee HY, Han DH, Lee JJ (2008) Electrical and optical properties of undoped p-type ZnO films. Surf Coat Technol 202(22–23):5463–5466
Lieberman M, Lichtenberg A (2005) Principles of plasma discharges and materials processing (2nd ed). Wiley Interscience, Hoboken, NJ
Caughey JMc, Kushner MJ (1989) J Appl Phys 65:186–195
Nikravech M, Baba K, Leneindre B, Rousseau F (2012) Role of reactive species in processing materials at laboratory temperature by spray plasma devices. Chem Pap 66(5):502–510
Ma Q-B, Ye Z–Z, He H-P, Zhu L-P, Wang J-R, Zhao B-H (2007) Influence of Ar/O2 ratio on the properties of transparent conductive ZnO:Ga films prepared by DC reactive magnetron sputtering. Mater Lett 61(11–12):2460–2463
Morita A, Watanabe I, Shirai H (2011) Chemical activity of oxygen atoms in the magnetron sputter-deposited ZnO films. Thin Solid Films 519(20):6903–6909
Khorsand Zak A, Abd. Majid WH, Abrishami ME, Yousefi R (2011) X-ray analysis of ZnO nanoparticles by Williamson-Hall and size–strain plot methods. Solid State Sci 13(1):251–256
Acknowledgments
This work was partially supported by the CNRS interdisciplinary energy program. Authors would like to thank Dr. Ovidiu Brinza for the help in Transmittance Electron Microscopy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baba, K., Lazzaroni, C. & Nikravech, M. Growth of ZnO Thin Films by Spray Plasma Technique: Correlation Between Spectroscopic Measurements and Film Properties. Plasma Chem Plasma Process 34, 1433–1446 (2014). https://doi.org/10.1007/s11090-014-9570-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-014-9570-0