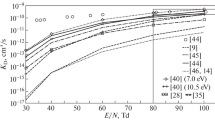
Abstract
One possible solution to mitigating the effects of high atmospheric concentrations of carbon dioxide (CO2) is the use of a plasma source to break apart the molecule into carbon monoxide (CO) and oxygen. This work experimentally investigates the efficiency of dissociation of CO2 in a 1-kW radio-frequency (rf) plasma source operating at 13.56-MHz in a low-pressure discharge. Mass spectrometry diagnostics are used to determine the species present in the discharge, and these measurements are used to calculate the energy efficiency and conversion efficiency of CO2 dissociation in the rf plasma source. Experimental results have found that the conversion efficiency of CO2 to CO can reach values near 90%, however energy efficiency reaches a maximum of 3%. A theoretical energy cost analysis is also given as a method to evaluate the effectiveness of any plasma system designed for CO2 emissions reduction.




Similar content being viewed by others
References
Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2007, U.S. EPA, April 2009
Dinan T (2007) Trade-offs in allocating allowances for CO2 emissions. Economic and Budget Issue Brief, Congressional Budget Office, April 27
Rubin ES, Rao AB (2002) A technical, economic and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control. U.S. Department of Energy, DOE/DE-FC26-00NT40935
Herzog H, Golomb D (2004) Carbon capture and storage from fossil fuel use. Encycl Energy 1–6:277–287
Varghese OK, Paulose M, LaTempa TJ, Grime CA (2009) High-rate solar photocatalytic conversion of CO2 and water vapor to hydrocarbon fuels. Nano Lett 9(2):731–737
Zheng G, Jiang J, Wu Y, Zhang R, Hou H (2003) The mutual conversion of CO2 and CO in dielectric barrier discharge (DBD). Plasma Chem Plasma Process 23(1):59–68
Oberreuther T, Wolff C, Behr A (2003) Volumetric plasma chemistry with carbon dioxide in an atmospheric pressure plasma using a technical scale reactor. IEEE Trans Plasma Sci 31(1):74–78
Tsuji M, Tanoue T, Nakano K, Nishimura Y (2001) Decomposition of CO2 into CO and O in a microwave-excited discharge flow of CO2/He or CO2/Ar mixtures. Chem Lett 1:22–23
Wu D, Outlaw RA, Ash RL (1996) Extraction of oxygen from CO2 using glow-discharge and permeation techniques. J Vac Sci Technol A 14(2):408–414
Wang J-Y, Xia G-G, Huang A, Suib SL, Hayashi Y, Matsumoto H (1999) CO2 decomposition using glow discharge plasmas. J Catal 185:152–159
Nguyen SVT, Foster JE, Gallimore AD (2009) Operating a radio-frequency plasma source on water vapor. Rev Sci Instrum 80
Fridman A (2008) Plasma chemistry. Cambridge University Press, New York ch. 5
Nguyen SVT (2009) Hydrogen production in a radio-frequency plasma source operating on water vapor. Ph.D. thesis, University of Michigan
Rusanov VD, Fridman AA, Sholin GV (1981) The physics of a chemically active plasma with nonequilibrium vibrational excitation of molecules. Sov Phys Usp 24:447–474
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spencer, L.F., Gallimore, A.D. Efficiency of CO2 Dissociation in a Radio-Frequency Discharge. Plasma Chem Plasma Process 31, 79–89 (2011). https://doi.org/10.1007/s11090-010-9273-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-010-9273-0