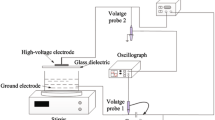
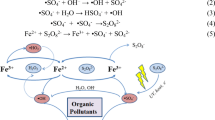
Abstract
A new plasma–catalysis process of gliding arc discharge (GAD) plasma with zero–valent iron (ZVI) was examined. Because GAD plasma creates an acidic environment, solid iron releases ferrous ions which act as a catalyst for the decomposition of the hydrogen peroxide. A comparative study of the catalytic effects between Fe2+ and Fe0 in GAD plasma was investigated. The decolorization reactions of Acid Orange 7 (AO7) followed pseudo–first–order kinetics. And the rate constants for the process of GAD with ZVI was increased by 30% and by 19%, respectively, compared with the process of GAD alone and the process of GAD with ferrous. The investigations of solution pH and hydrogen peroxide both demonstrated that the GAD plasma induced conditions are much suitable for advanced Fenton reactions. The corrosion of ZVI in GAD plasma can give continuous ferrous ions to sustain Fenton reaction. Also, ZVI was demonstrated to have favorable reusable feature.













Similar content being viewed by others
References
Brown MA, De Vito SC (1993) Crit Rev Environ Sci Technol 23:249
Ramirez JH, Duarte FM, Martins FG, Costa CA, Madeira LM (2009) Chem Eng J 148:394
Velegraki T, Poulios I, Charalabaki M, Kalogerakis N, Samaras P, Mantzavinos D (2006) Appl Catal B Environ 62:159
Hammami S, Bellakhal N, Oturan N, Oturan MA, Dachraoui M (2008) Chemosphere 73:678
Nakano Y, Okawa K, Nishijima W, Okada M (2003) Water Res 37:2595
Chen Y, Yang S, Wang K, Lou L (2005) J Photochem Photobiol A 172:47
Mok YS, Jo J-O, Whitehead JC (2008) Chem Eng J 142:56
Zhang S–J, Yu H–Q, Zhao Y (2005) Water Res 39:839
Inoue M, Okada F, Sakurai A, Sakakibara M (2006) Ultrason Sonochem 13:313
Truong GL, Laat JDD, Legube B (2004) Water Res 38:2384
Czenichovski A (1994) Pure Appl Chem 66:1301
Abdelmalek F, Torres RA, Combet E, Petrier C, Pulgarin C, Addou A (2008) Sep Purif Technol 63:30
Yan JH, Du CM, Li XD, Sun XD, Ni MJ, Cen KF, Cheron B (2005) Plasma Sources Sci Technol 14:637
Yu L, Li X, Tu X, Wang Y, Lu S, Yan J (2009) J Phys Chem A 114:360
Rivas FJ, Carbajo M, Beltrán F, Gimeno O, Frades J (2008) J Hazard Mater 155:407
Wang H, Li J, Quan X, Wu Y (2008) Appl Catal B Environ 83:72
Wang Y, Zhao D, Ma W, Chen C, Zhao J (2008) Environ Sci Technol 42:6173
Feng J, Hu X, Yue PL (2003) Environ Sci Technol 38:269
Tezcanli–Güyer G, Ince NH (2004) Ultrasonics 42:603
Sunka P (2001) Phys Plasmas 8:2587
Ghezzar MR, Abdelmalek F, Belhadj M, Benderdouche N, Addou A (2007) Appl Catal B Environ 72:304
Maroulf–Khelifa K, Abdelmalek F, Khelifa A, Addou A (2008) Chemosphere 70:1995
Burlica R, Locke B (2009) Gliding arc electrical discharge reactors with improved nozzle configuration, US 2009
Du CM, Sun YW, Zhuang XF (2008) Plasma Chem Plasma Process 28:523
Burlica R, Kirkpatrick MJ, Locke BR (2006) J Electrostat 64:35
Koprivanac N, Kusic H, Vujevic D, Peternel I, Locke BR (2005) J Hazard Mater 117:113
Hao X, Zhou M, Xin Q, Lei L (2007) Chemosphere 66:2185
Mededovic S, Locke BR (2007) Appl Catal B Environ 72:342
Zhang H, Zhang J, Zhang C, Liu F, Zhang D (2009) Ultrason Sonochem 16:325
Bremner DH, Burgess AE, Houllemare D, Namkung K–C (2006) Appl Catal B Environ 63:15
Anotai J, Lu M–C, Chewpreecha P (2006) Water Res 40:1841
Namkung KC, Burgess AE, Bremner DH, Staines H (2008) Ultrason Sonochem 15:171
Chakinala AG, Bremner DH, Gogate PR, Namkung K–C, Burgess AE (2008) Appl Catal B Environ 78:11
Du CM, Shi TH, Sun YW, Zhuang XF (2008) J Hazard Mater 154:1192
Benstaali B, Boubert P, Cheron B, Addou A, Brisset J (2002) Plasma Chem Plasma Process 22:553
Eisenberg G (1943) Ind Eng Chem Anal Ed 15:327
Kallel M, Belaid C, Mechichi T, Ksibi M, Elleuch B (2009) Chem Eng J 150:391
Nam S, Tratnyek PG (2000) Water Res 34:1837
Zhang H, Duan L, Zhang Y, Wu F (2005) Dyes Pigments 65:39
Özcan A, Oturan MA, Oturan N, Sahin Y (2009) J Hazard Mater 163:1213
Ghezzar MR, Abdelmalek F, Belhadj M, Benderdouche N, Addou A (2009) J Hazard Mater 164:1266
Yan JH, Liu YN, Bo Z, Li XD, Cen KF (2008) J Hazard Mater 157:441
Gao J, Liu Y, Yang W, Pu L, Yu J, Lu Q (2003) Plasma Sources Sci Technol 12:533
Duesterberg CK, Mylon SE, Waite TD (2008) Environ Sci Technol 42:8522
Jeong J, Yoon J (2005) Water Res 39:2893
Tang WZ, Chen RZ (1996) Chemosphere 32:947
Wang L, Jiang X (2009) J Hazard Mater 161:926
Duesterberg CK, Waite TD (2006) Environ Sci Technol 40:4189
Gotpagar J, Lyuksyutov S, Cohn R, Grulke E, Bhattacharyya D (1999) Langmuir 15:8412
Zhou T, Li Y, Ji J, Wong F–S, Lu X (2008) Sep Purif Technol 62:551
Huang YH, Zhang TC (2005) Water Res 39:1751
Acknowledgments
The project is supported by the National Nature Science Foundation (50908237, 20977117), Specialized Research Fund for Doctoral Program of Higher Education of China (200805581036), Guangdong Provincial Nature Science Foundation (845102750100150, 92510027501000005), Fundamental Research Funds for the Central Universities (09lgpy21) and Project of New Technology and Process of Guangzhou EPA (2009–03).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Du, C., Zhang, L., Wang, J. et al. Degradation of Acid Orange 7 by Gliding Arc Discharge Plasma in Combination with Advanced Fenton Catalysis. Plasma Chem Plasma Process 30, 855–871 (2010). https://doi.org/10.1007/s11090-010-9249-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-010-9249-0