Abstract
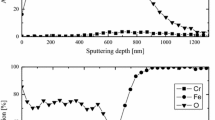
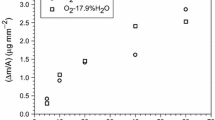
Detailed microstructure investigations were performed on oxide scales formed on 310 stainless steel exposed isothermally at 600 °C to O2 with 40% water vapour for 1–336 h. FIB microscopy was used to study the evolution of the surface morphology and to prepare cross-section TEM thin foils of the oxide scales. The foils were investigated by analytical transmission electron microscopy. The results showed that a thin protective base oxide scale had formed after 1 h. Due to Cr loss from the oxide scale through water vapour induced Cr evaporation, local breakaway oxidation occurs, resulting in the formation of oxide nodules. The development of these nodules depends on whether a new Cr-rich healing layer is formed or not. A model for the evolution of the oxide scale is proposed based on the results regarding the composition and distribution of various phases in the oxide scale and subjacent steel.
























Similar content being viewed by others
References
W. H. Hatfield, in Iron and Steel Institute—Meeting, Vol. 9 (Iron and Steel Institute, 1927), p. 26.
C. T. Fujii and R. A. Meussner, Journal of Electrochemical Society 111, 1215 (1964).
I. Kvernes, M. Oliveira, and P. Kofstad, Corrosion Science 17, 237 (1977).
P. Kofstad, in High Temperature Corrosion, Elsevier Applied Science, Chapter 11 (1988), pp. 342–387.
D. Caplan and M. Cohen, Journal of Electrochemical Society 108, 438 (1961).
D. Caplan and M. Cohen, Corrosion 15, 141t (1959).
A. Rahmel and J. Tobolski, Corrosion Science 5, 333 (1965).
M. Thiele, H. Teichmann, W. Schwarz, and W. J. Quadakkers, VGB Kraftwerkstechnik 2, 129 (1997).
A. Holt and P. Kofstad, Solid State Ionics 69, 137 (1994).
S. Jiannian, Z. Lingjiang, and L. Tiefan, Oxidation of Metals 48, 347 (1997).
W. J. Quadakkers and P. J. Ennis, VGB Kraftwerkstechnik 2, 123 (1997).
H. Asteman, J.-E. Svensson, L.-G. Johansson, and M. Norell, Oxidation of Metals 52, 95 (1999).
H. Asteman, J.-E. Svensson, and L.-G. Johansson, Oxidation of Metals 57, 193 (2002).
K. Segerdal, J.-E. Svensson, and L.-G. Johansson, Materials and Corrosion 53, 247 (2002).
K. Segerdal, J.-E. Svensson, and L.-G. Johansson, Materials and Corrosion 53, 479 (2002).
H. Asteman, K. Segerdal, J.-E. Svensson, and L.-G. Johansson, Materials Science Forum 369–372, 277 (2001).
H. Asteman, J.-E. Svensson, and L.-G. Johansson, Corrosion Science 44, 2635 (2002).
A. Yamauchi, K. Kurokawa, and H. Takahashi, Oxidation of Metals 59, 517 (2003).
E. Opila, Materials Science Forum 461–464, 765 (2004).
G. C. Wood and D. P. Whittle, Corrosion Science 7, 763 (1967).
J. E. Tang, F. Liu, H. Asteman, J.-E. Svensson, L.-G. Johansson, and M. Halvarsson, Materials at High Temperatures 24, 27 (2007).
I. Saeki, T. Saito, R. Furuichi, H. Konno, T. Nakamura, K. Mabuchi, and M. Itoh, Corrosion Science 40, 1295 (1998).
X. Peng, J. Yan, Y. Zhou, and F. Wang, Acta Materialia 53, 5079 (2005).
S. N. Basu and G. J. Yurek, Oxidation of Metals 36, 281 (1991).
G. J. Yurek, D. Eisen, and A. Garratt-Reed, Metallurgical and Materials Transactions A 13, 473 (1982).
D. R. Baer and M. D. Merz, Metallurgical Transactions A 11A, 1973 (1980).
M. Halvarsson, J. E. Tang, H. Asteman, J.-E. Svensson, and L.-G. Johansson, Corrosion Science 48, 2014 (2006).
H. E. Evans, A. T. Donaldson, and T. C. Gilmour, Oxidation of Metals 52, 379 (1999).
H. E. Evans, D. A. Hilton, R. A. Holm, and S. J. Webster, Oxidation of Metals 14, 235 (1980).
F. Liu, J. E. Tang, T. Jonsson, S. Cnovic, K. Segerdal, J.-E. Svensson, and M. Halvarsson, Oxidation of Metals 66, 295 (2006).
Acknowledgements
This work was carried out within the Swedish High Temperature Corrosion centre (HTC) with financial support partly provided by the Swedish National Research Council (VR).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, F., Tang, J.E., Asteman, H. et al. Investigation of the Evolution of the Oxide Scale Formed on 310 Stainless Steel Oxidized at 600 °C in Oxygen with 40% Water Vapour Using FIB and TEM. Oxid Met 71, 77–105 (2009). https://doi.org/10.1007/s11085-008-9130-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-008-9130-1