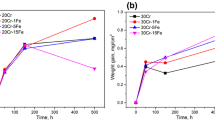
The oxidation behavior of Ni and Ni-3, 6, and 10Al alloys at 800°C in an N2–O2 gas mixture was investigated. The mass gain of each alloy depended on both the oxidation periods and Al content. NiO scale was formed on all alloy substrates accompanied by internal oxides of Al2O3. Many cavities were formed at the NiO/substrate interface at shorter oxidation times, and these cavities were found to be filled by metallic Ni(Al) from the matrix in the internal-oxidation zone by the development of internal oxides. The filling of cavities by Ni(Al) was more significant on higher Al alloys, which had a higher density of internal Al2O3. Once metallic Ni(Al) formed along the entire NiO/substrate interface, the oxidation kinetics became the same as pure Ni. It was concluded that pure Ni filling the cavities at the interface provided a diffusion path of Ni from the substrate to the NiO scale, and that controlled the oxidation kinetics.










Similar content being viewed by others
References
Pettit F. S. (1967) Transactions of the Metallurgical Society of AIME 239:1296
Hindam H. M., Smeltzer W. W. (1980) Journal of Electrochemical Society 127:1622
Scott F. H., Wood G. C. (1977) Corrosion Science 17:647
Wolf J., Evans E. B. (1962) Corrosion 18:129
Elefaie F. A., Smeltzer W. W. (1982) Oxidation of Metals 17:407
Giggins C. S., Pettit F. S. (1971) Journal of Electrochemical Society 118:1782
Goto S., Koda S. (1968) Journal of the Japan Institute of Metals 34:334
Hagel W. C. (1965) Corosion 21:316
Nesbitt J. A. (1989) Journal of Electrochemical Society 5:1511
Shida Y., Stott F. H., Bastow B. D., Whittle D. P., Wood G. C. (1982) Oxidation of Metals 18:93
Fueki K., Ishibashi H. (1961) Journal of Electrochemical Society 108:306
Whittle D. P., et al (1982) Philosophical Magazine A 46:931
Martinez-Villafane A., et al (2002) Oxid. Met. 57:267
Mackrt J. R. Jr., Ringle R. D., Fairhurst C. W. (1983) Journal of Dental Research 62:1229
Savva G. C., Weatherly G. C., Kirkaldy J. S. (1996) Scripta Materialia 34:1087
Stott F. H., Shida Y., Whittle D. P., Wood G. C., Bastow B. D. (1982) Oxidation of Metals 18:127
Yi H. C., Guan S. W., Smeltzer W. W., Petric A. (1994) Acta Metallurgica et Materialia 42:981
Gesmundo F., Gleeson B. (1995) Oxidation of Metals 44: 211–236
Wang G., Gleeson B., Douglass D. L. (1991) Oxidation of Metals 35:333
Young D., Gleeson B. (2002) Corrosion Science 44:345
Carter P., Gleeson B., Yonug D. J. (1996) Acta Materialia 44:4033
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hayashi, S., Narita, S. & Narita, T. Oxidation Behavior of Ni-3,6, 10 wt.% Al Alloys at 800°C. Oxid Met 66, 191–207 (2006). https://doi.org/10.1007/s11085-006-9034-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-006-9034-x