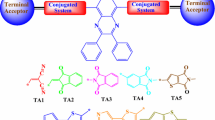
Abstract
The third order nonlinear optical properties were reported for a new synthesized molecules based on triazepine and thianine, using the third harmonic generation (THG) technique. After synthesis, the powders were deposited on glass substrates using dip coating technique in order get thin films. These thin films were characterized, by different techniques, we mention the absorption and X-ray diffraction. The measurements of third order nonlinear optical susceptibilities were performed on these thin films, using the THG technique at 1064 nm. These investigations were completed by theoretical studies, using energy levels theory HOMO–LUMO and the second order hyperpolarizabilities (γ) results. Good agreement was obtained between the theoretical and experimental results.










Similar content being viewed by others
References
Achelle, S.: Pyrimidine ring as building block for the synthesis of functionalized π-conjugated materials. Curr. Org. Synth. 9, 163–187 (2012)
Achelle, S., Baudequin, C.: Recent advances in pyrimidine derivatives as luminescent, photovoltaic and ono-linear optical materials. Targets Heterocycl. Syst. 17, 1–34 (2013)
Achelle, S., Kahlal, S., Barsella, A., Saillard, J.-Y., Che, X., et al.: Improvement of the quadratic non-linear optical properties of pyrimidine chromophores by N-methylation and tungsten pentacarbonyl complexation. Dyes Pigments 113, 562–570 (2015)
Ahluwalia, V.K., Batla, R., Khuranaand, A., Kumar, R.: Synthesis of 1,3-diaryl-1,2,3,4-tetrahydro-7,7-diethyl-5-methyl-4-oxo-2-thioxo- 7H- pyrano(2,3-d)pyrimidines. Indian J. Chem. 29B, 1141 (1990)
Apostoluk, A., Chapron, D., Gadret, G., Sahraoui, B., Nunzi, J.-M., Fiorini-Debuisschert, C., Raimond, P.: Quasi-phase-matched gratings printed by all-optical poling in polymer films. Opt. Lett. 27(22), 2028–2030 (2002)
Azarifar, D., Nejat-Yami, R., Sameriand, F., Akrami, A.: Ultrasonic-promoted one-pot synthesis of 4H-chromenes, pyrano[2,3- d]pyrimidines, and 4H-pyrano[2,3-c]pyrazoles. Lett. Org. Chem. 9(6), 435–439 (2012)
Becke, D.: Density-functional thermochemistry. III, the role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993)
Bogaard, M.P., Orr, B.J., Buckingham, A.D. (Ed.): MTP International Review of Science, vol. 2, pp. 149–194. Butterworths, London (1975)
Brown, R., Pomp, A., Hart, C.M., Deleeuw, D.M.: Logic gates made from polymer transistors and their use in ring oscillators. Science 270, 972–974 (1995)
Burroughes, J.H., Bradley, D.D.C., Brown, A.R., Marks, R.N., MacKay, K., Friend, R.H., Burn, P.L., Holmes, A.B.: Light-emitting diodes based on conjugated polymers. Nature 347, 539–541 (1990)
Cave, R.J., Burke, K., Castner, E.W.: Theoretical investigation of the ground and excited states of coumarin 151 and coumarin 120. J. Phys. Chem. A 106, 9294–9305 (2002)
Derkowska, B., Mulatier, J.C., Fuks, I., Sahraoui, B., Nguyen Phu, X., Andraud, C.: Third-order optical nonlinearities in new octupolar molecules and their dipolar subunits. JOSA B 18(5), 610–616 (2001)
Devi, I., Kumar, B.S.D., Bhuyan, P.J.: A novel three-component one-pot synthesis of pyrano[2,3-d]pyrimidines and pyrido[2,3-d]pyrimidines using microwave heating in the solid state. Tetrahedron Lett. 44(45), 8307–8310 (2003)
Fuuya, S., Ohtaki, T.: Pyrido[2,3-d]pyrimidines and their uses as anatagonists, Eur. Patent, 608565, Chem. Abstr. 121, 205395, (1994)
Gao, S.J., Tsai, C.H., Tsengand, C., Yao, C.F.: Fluoride ion catalyzed multicomponent reactions for efficient synthesis of 4H-chromene and N-arylquinoline derivatives in aqueous media. Tetrahedron 64(38), 9143–9149 (2008)
Gaussian 09: Revision D.01, Gaussian, Inc., Wallingford CT, Official Gaussian 09 Literature Citation (2009)
Grivaky, E.M., Lee, S., Siyal, C.W., Duchand, D.S., Nichol, C.A.: Synthesis and antitumor activity of 2,4-diamino-6-(2,5-dimethoxybenzyl)-5-methylpyrido[2,3-d]pyrimidine. J. Med. Chem. 23(3), 327–329 (1980)
Heber, D., Heersand, C., Ravens, U.: Positive inotropic activity of 5-amino-6-cyano-1,3-dimethyl-1,2,3,4-tetrahydropyrido[2,3-d]pyrim idine-2,4-dione in cardiac muscle from guinea-pig and man. Part 6: compounds with positive inotropic activity. Die Pharm. 48(7), 537–541 (1993)
Khan, A.T., Lal, M., Aliand, S., Khan, M.M.: One-pot three-component reaction for the synthesis of pyran annulated heterocyclic compounds using DMAP as a catalyst. Tetrahedron Lett. 52(41), 5327–5332 (2011)
Khazaei, A., Ranjbaran, A., Abbasi, F., Khazaei, M., Reza Moosavi-Zare, A.: Synthesis, characterization and application of ZnFe2O4 nanoparticles as a heterogeneous ditopic catalyst for the synthesis of pyrano[2,3-d] pyrimidines. RSC Adv. 5, 13643–13647 (2015)
Khurana, J.M., Vij, K.: Nickel nanoparticles as semiheterogeneous catalyst for one-pot, three-component synthesis of 2-amino-4H-pyrans and pyran annulated heterocyclic moieties. Synth. Commun. 43(17), 2294–2304 (2013)
Khurana, J.M., Nandand, B., Saluja, P.: DBU: a highly efficient catalyst for one-pot synthesis of substituted 3,4-dihydropyrano[3,2-c]chromenes, dihydropyrano-[4,3-b] pyranes, 2-amino-4Hbenzo[h]chromenes and 2-amino-4H benzo[g]chromenes in aqueous medium. Tetrahedron 66(30), 5637–5641 (2010)
Kolev, T., Kityk, I.V., Ebothe, J., Sahraoui, B.: Intrinsic hyperpolarizability of 3-dicyanomethylene-5,5-dimethyl-1-[2-(4-hydroxyphenyl)ethenyl]-cyclohexene nanocrystallites incorporated into the photopolymer matrices. Chem. Phys. Lett. 443(4–6), 309–312 (2007)
Kraft, A., Grimsdale, A.C., Holmes, A.B.: Electroluminescent conjugated polymers—seeing polymers in a new light. Angew. Chem. Int. Ed. 37, 402–428 (1998)
Li, L., Ge, J., Wu, H., Xu, Q.H., Yao, S.Q.: Organelle-specific detection of phosphatase activities with two-photon fluorogenic probes in cells and tissues. J. Am. Chem. Soc. 134, 12157–12167 (2012)
Lian, X.-Z., Huang, Y., Li, Y.-Q., Zheng, W.-J.: A green synthesis of tetrahydrobenzo[b]pyran derivatives through three-component condensation using N-methylimidazole as organocatalyst. Monatsh. Chem. 139(2), 129–131 (2008)
Liu, B., Hu, X.L., Liu, J., Zhao, Y.D., Huang, Z.L.: Synthesis and photophysical properties of novel pyrimidine-based two-photon absorption chromophores. Tetrahedron Lett. 48, 5958–5962 (2007)
Migalska-Zalas, A., Sofiani, Z., Sahraoui, B., Kityk, I.V., Tkaczyk, S., Yuvshenko, V., Fillaut, J.-L., Perruchon, J., Muller, T.J.J.: χ(2) Grating in Ru Derivative Chromophores Incorporated within the PMMA Polymer Matrices. J. Phys. Chem. B 108(39), 14942–14947 (2004)
Sahraoui, B., Nguyen Phu, X., Sallé, M., Gorgues, A.: Electronic and nuclear contributions to the third-order nonlinear optical susceptibilities of new p-N, N′-dimethylaniline tetrathiafulvalene derivatives. Opt. Lett. 23(23), 1811–1813 (1998)
Sheats, J.R., Antoniadis, H., Hueschen, M., Leonard, W., Miller, J., Moon, R., Roitman, D., Stocking, A.: Effect of heat extraction by metal lines and two sided cooling on temperatures in organic light emitting diode based devices. Science 273, 884–888 (1996)
Shen, J., Cheng, W.-D., Wu, D.-S., Li, X.-D., Lan, Y.-Z., Zhang, H., Gong, Y.-J., Li, F.-F., Huang, S.-P.: Modeling of configurations and third-order nonlinear optical properties of methyl silsesquioxanes. J. Chem. Phys. 122, 20470–204709 (2005)
Sofiani, Z., Derkowska, B., Dalasiński, P., Wojdyła, M., Dabos-Seignon, S., AlaouiLamrani, M., Dghoughi, L., Bała, W., Addou, M., Sahraoui, B.: Optical properties of ZnO and ZnO: Ce layers grown by spray pyrolysis. Opt. Commun. 267(2), 433–439 (2006)
Tessler, N., Denton, G.J., Friend, R.H.: Lasing from conjugated-polymer microcavities. Nature 382, 695–697 (1996)
Yu, G., Heeger, A.J.: Charge separation and photovoltaic conversion in polymer composites with internal donor/acceptor heterojunctions. J. Appl. Phys. 78, 4510–4515 (1995)
Yu, G., Gao, J., Hummelen, J.C., Wudl, F., Heeger, A.J.: Polymer photovoltaic cells: enhanced efficiencies via a network of internal donor–acceptor heterojunctions. Science 270, 1789–1791 (1995)
Acknowledgments
This work was supported by “Académie Hassan II des Sciences et Techniques”, Rabat, Morocco. Calculations have been carried out in Wroclaw Centre for Networking and Supercomputing (http://www.wcss.pl), Grant No. 282.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This article is part of the Topical Collection on Advanced Materials for Photonics and Electronics.
Guest Edited by Bouchta Sahraoui, Yahia Boughaleb, Kariem Arof, Anna Zawadzka.
Rights and permissions
About this article
Cite this article
Sofiani, Z., Khannyra, S., Boucetta, A. et al. Nonlinear optical properties of new synthesized conjugated organic molecules based on pyrimidine and oxazepine. Opt Quant Electron 48, 282 (2016). https://doi.org/10.1007/s11082-016-0549-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-016-0549-3