Abstract
It is important to find new treatments for addiction due to high relapse rates despite current interventions and due to expansion of the field with non-substance related addictive behaviors. Neuromodulation may provide a new type of treatment for addiction since it can directly target abnormalities in neurocircuits. We review literature on five neuromodulation techniques investigated for efficacy in substance related and behavioral addictions: transcranial direct current stimulation (tDCS), (repetitive) transcranial magnetic stimulation (rTMS), EEG, fMRI neurofeedback and deep brain stimulation (DBS) and additionally report on effects of these interventions on addiction-related cognitive processes. While rTMS and tDCS, mostly applied at the dorsolateral prefrontal cortex, show reductions in immediate craving for various addictive substances, placebo-responses are high and long-term outcomes are understudied. The lack in well-designed EEG-neurofeedback studies despite decades of investigation impedes conclusions about its efficacy. Studies investigating fMRI neurofeedback are new and show initial promising effects on craving, but future trials are needed to investigate long-term and behavioral effects. Case studies report prolonged abstinence of opioids or alcohol with ventral striatal DBS but difficulties with patient inclusion may hinder larger, controlled trials. DBS in neuropsychiatric patients modulates brain circuits involved in reward processing, extinction and negative-reinforcement that are also relevant for addiction. To establish the potential of neuromodulation for addiction, more randomized controlled trials are needed that also investigate treatment duration required for long-term abstinence and potential synergy with other addiction interventions. Finally, future advancement may be expected from tailoring neuromodulation techniques to specific patient (neurocognitive) profiles.
Similar content being viewed by others
Introduction
Addiction is a burdensome public health concern that not only affects the addicted individual but also greatly impacts his or her family members and surroundings. Though several treatment options are available, many patients show a chronic course of addiction with abstinence rates between 40% and 60% 1-year post treatment (Mclellan et al. 2000). In the last few decades there is more awareness for the problem of nonsubstance related addictions such as gambling disorder (reclassified as an addictive disorder in 5th edition of the diagnostic and statistical manual of mental disorders) and excessive internet use and gaming, increasing the importance of finding new and better interventions for addiction (Banz et al. 2016).
In recent years our neurobiological knowledge of addiction has expanded. We know more about changes in brain structure, function and its neurochemistry (Fineberg et al. 2010; Koob 2015; Koob and Volkow 2009; Volkow et al. 2012). Yet, the translation of this knowledge into new interventions or improvement of existing interventions has fallen short. The non-selective manner in which effective psychotherapeutic and pharmacological interventions induce brain changes makes it difficult to apply neurobiological knowledge to improve these interventions. Neuromodulation may close the gap by targeting directly abnormalities in brain functioning. Direct intervention in the brain in order to change addiction is not new, on the contrary, neurosurgery for addiction has a long and turbulent history (Stelten et al. 2008). Yet, the advances in our understanding of brain function and the developments of new neuromodulation techniques that are non-invasive or reversible nowadays have made this intervention method a safer and more promising option.
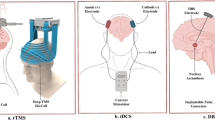
Repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) have been used most frequently for treatment of addiction. Other neuromodulatory options for addiction are deep brain stimulation (DBS), and neurofeedback which can be applied with Electroencephalography (EEG neurofeedback) or functional magnetic resonance imaging (fMRI neurofeedback). These techniques have been investigated as stand-alone or add on treatment and while some have been around for decades (EEG neurofeedback), others have just emerged as promising intervention for addiction (fMRI neurofeedback). In this review we will evaluate, compare and discuss the experimental application of these neuromodulation techniques for treatment of addiction.
A complicating factor in the application of neuromodulation for addiction is its heterogeneity, with large individual differences in underlying brain abnormalities (George and Koob 2010). Therefore, we choose to not only review how neuromodulation affects primary treatment outcomes for addiction such as craving or relapse, but additionally focus on how neuromodulation affects different cognitive processes involved in addiction. Cognitive processes such as reward processing, cognitive control and emotion regulation may constitute different factors underlying addiction with a closer mapping to neural abnormalities than the currently classified symptoms of addiction. One could envision that using this approach, an individually tailored type of neuromodulation that fits the cognitive profile of the patient would become possible. For instance, a highly impulsive addicted patient may receive neuromodulation focused on enhancing cognitive control in prefrontal regions whereas deeper regions like the insula or amygdala might be targeted in a patient whose addiction is driven by harm avoidance. Although the number of studies investigating the effect of neuromodulation on these cognitive processes in addiction is very limited, we will discuss them where possible.
This review aims to discuss the efficacy of neuromodulation techniques for the treatment of addiction. Although still in an early phase of development, we additionally review neuromodulatory induced cognitive changes relevant for addiction. As addictions are perpetuated by numerous maladaptive cognitive processes that can be influenced with neuromodulation techniques, this is a highly promising area for future research and treatment development. This review extends upon existing reviews addressing rTMS, tDCS (Hone-Blanchet et al. 2015; Jansen et al. 2013; Lupi et al. 2017) with additional DBS for addiction (Coles et al. 2018; Spagnolo and Goldman 2017) by (1) specifically considering only the highest quality evidence for each technique in order to evaluate the potential for clinical efficacy, (2) expanding the definition of neuromodulation to consider fMRI and EEG neurofeedback, and (3) reviewing neuromodulatory impact on addiction-related cognitive processes and discussing potential future neurocognitive targets.
Methods
Our review focusses on studies that included adult participants who were treated for substance or behavioral addictions with neuromodulation (i.e., rTMS, tDCS, DBS, EEG & fMRI neurofeedback). With this aim we searched MEDLINE databases using PubMed for the indication terms: (addiction OR substance disorder OR craving OR gambling disorder OR binge eating disorder OR bulimia OR smoking) AND (transcranial magnetic stimulation OR repetitive transcranial magnetic stimulation OR transcranial direct current stimulation OR tDCS OR neurofeedback OR deep brain stimulation). The reference lists of relevant papers were inspected for further studies that fit the inclusion criteria. The search was conducted on the literature before August 2017 without historic limitation, and only studies written in English were included. As there are large differences in number, quality and methodologies of studies between the neuromodulation techniques we used specific inclusion criteria to identify the highest quality of available evidence for each technique where we prioritize (1) sham-controlled designs (2) outcome measures focused on clinical efficacy (3) number of sessions for non-chronic neuromodulation techniques (4) and number of participants. Because of the large differences in quality and quantity of studies between the modulation techniques, we will outline which of the criteria will be applied at the beginning of each section below.
Results
Non-Invasive Brain Stimulation (TMS & tDCS)
Non-invasive brain stimulation techniques are growing in popularity as they offer a safe, economical and increasingly accessible means of modulating neural activity. The two most common and best developed methods are rTMS and tDCS. The majority of studies investigating rTMS and tDCS for addiction are single session experimental investigations that incorporate a vast array of stimulation parameters, participant characteristics, and outcome measures. The results of these studies are heterogeneous and do little to determine clinical efficacy. Of the small number of trials delivering multi-session stimulation many lack a sham condition and rely only on self-report measures, which is problematic as this does not control for placebo response or desirability reporting. Therefore, we focus exclusively on studies that meet the following criteria:
-
a.
Five or more stimulation sessions
-
b.
Include a sham stimulation comparator condition,
-
c.
Deliver stimulation with the express aim of modifying craving and/or frequency of engaging in addictive behaviors.
We note that a course of >5 stimulation sessions is a substantially lesser dose than the 20–30 sessions typically considered a therapeutic course for psychiatric indications such as major depression. However, as addiction trials have delivered acute courses ranging from 5 to 13 stimulation sessions this threshold is reflective of the current state of clinical research in this area.
Transcranial Direct Current Stimulation
tDCS involves application of a low voltage (typically 0.5 mA – 2 mA) electrical current to the scalp via surface electrodes. Current flows in one direction from a positive anode electrode to a negative cathode electrode. There are many research trials investigating tDCS, likely because of its ease of administration, low side effect profile and low cost. The strength and direction of neuromodulation effects are determined by the density, duration and direction of the current that penetrates the skull and comes into contact with underlying neurons (Batsikadze et al. 2013; Jamil et al. 2017; Rawiji et al. 2017). The impact of tDCS on neuronal activity arises from two mechanisms: 1. initial sub-threshold depolarization or hyperpolarization of neuronal membrane potentials which increases or decreases (respectively) the likelihood of spontaneous neuronal firing and 2. (following prolonged / repeated stimulation) facilitation of long-term potentiation or long-term depression like synaptic plasticity (Woods et al. 2016). Proposedly anodal stimulation enhances cortical excitability via depolarization and long-term potentiation whereas cathodal stimulation inhibits excitability via hyperpolarization and long-term depression (Nitsche and Paulus 2000). It is noteworthy, however, that the effects of anodal and cathodal stimulation vary considerably depending on stimulation protocols and inter-individual differences in neurophysiology, clinical status and cognitive capacity (Batsikadze et al. 2013; Jamil et al. 2017; Strube et al. 2016).
tDCS for addictions has been investigated in 7 sham-controlled trials. All stimulated the dorsolateral prefrontal cortex (DLPFC) (see Table 1). Four investigated bilateral DLPFC stimulation, two manipulated current density and flow to favor the left DLPFC and one to the right (i.e. by applying a larger or extra-cephalic return electrode). The rationale for stimulating the DLPFC is based on three observations: 1. The DLPFC has been implicated in spontaneous and cue elicited craving, 2. The DLPFC is involved in decision making, inhibitory control, attentional bias, and awareness, 3. Stimulation of the DLPFC may affect the reward circuitry via efferents to the nucleus accumbens (NA) and ventral tegmental area (VTA).
Two controlled trials have investigated the impact of tDCS on cigarette craving and consumption (Table 1). Boggio et al. (2009) used a cross-over design to compare craving in response to visual smoking cues and cigarette consumption during a course of five consecutive daily sessions of left DLPFC anodal tDCS. Active stimulation was associated with greater reduction in self-reported craving intensity and number of cigarettes consumed (median 30% reduction in cigarettes smoked during active tDCS versus 10% reduction during sham), however the integrity of stimulation blinding was not assessed, and no objective outcome measures used. Fecteau et al. (2014) focused on aspects of decision making that contribute to smoking behaviors. Also using a five-session (over 5 consecutive days) cross-over design and cue-reactivity paradigm they found that, relative to sham, bilateral DLPFC tDCS reduced number of cigarettes consumed by 3–4 per day, decreased reported desire to smoke in response to cues, but did not modulate other aspects of craving. Moreover, trend level reduction in exhaled carbon monoxide was observed. Interestingly, using the Ultimatum Game this study found that participants rejected more offers of cigarettes following active tDCS whereas responses to monetary offers were unchanged indicating that motivation for cigarettes changed while preserving motivation for non-drug rewards.
Two tDCS trials conducted in alcohol dependence both delivered stimulation in addition to routine outpatient treatment (Table 1). They differed in tDCS protocol and therapeutic outcomes. Klauss et al. (2014) tested bilateral DLPFC tDCS and applied two short stimulation sessions separated by 20-min interval (a protocol shown to induce prolonged excitatory after effects in the motor cortex; Monte-Silva et al. 2013) across five consecutive days. Both active and sham tDCS were associated with reduced craving for alcohol, mild decrease in anxiety and increase in DLPFC mediated cognitive functions assessed by the Frontal Assessment Battery (FAB). At six-month follow up, however, a significantly lower relapse rate was reported for participants who had undergone active tDCS, with 50% having returned to regular heavy drinking compared to 88% in the sham condition. In contrast, da Silva et al. (2013) applied one session of active vs sham left anodal tDCS per week for five weeks and monitored participants for four further weeks post-stimulation. Active tDCS was associated with acute reduction in craving, depressive severity and density of prefrontal event-related potential responses to both alcohol-related and neutral visual cues, and tDCS did not impact anxiety or performance on the FAB. Sixty-seven percent of participants in the active condition and 14% in the sham condition relapsed during the program. While this pattern appears suggestive of an iatrogenic effect of active tDCS, given the infrequent stimulation schedule it is unlikely that this is the case. The effects of a single tDCS session wear off within hours, making the cumulative impact of one tDCS session per week negligible (Monte-Silva et al. 2013). Indeed, experimental tDCS studies typically use between session wash-out periods of one week, and as such it is difficult to attribute either positive or negative observations in this study to tDCS (Stagg and Nitsche 2011; Woods et al. 2016).
The impact of tDCS on addiction to crack cocaine has been studied in two controlled trials, both of which delivered right anodal and left cathodal DLPFC stimulation (Table 1). Batista et al. (2015) applied active or sham tDCS every second day for ten days. While active stimulation was associated with reduction of craving, depression and anxiety, the efficacy of tDCS blinding was not reported and the study only used subjective outcome measures. Conversely, Conti and Nakamura-Palacios (2014) performed one of the few trials to include a biological outcome measure: following five consecutive daily stimulation sessions there was a significant decrease in anterior cingulate cortex reactivity during exposure to crack cocaine cues following active but not sham tDCS.
There is a dearth of research into tDCS for behavioral addictions. Only a single trial has been conducted into food craving in healthy individuals, specifically excluding those with a history of binge eating or other eating disorder, which did not report on changes in eating behavior (Ljubisavljevic et al. 2016; Table 1). Significant reduction in food craving was documented after an initial active session of right anodal DLPFC tDCS, with superior outcome for active relative to sham tDCS maintained following four further tDCS sessions and at 30-day follow up. Integrity of the sham control was verified by asking participants to guess their stimulation condition at the end of the tDCS course, and a majority (>85%) in both conditions believed they received active stimulation.
Overall, the results of controlled trials of tDCS for addiction are inconclusive. While modest positive effects on craving have been reported by a number of studies findings are inconsistent and the short courses of DLPFC stimulation applied to date have not been associated with prominent or persistent behavioral change. In order to evaluate the potential of tDCS for addiction, trials delivering longer courses of stimulation, prioritizing long-term outcomes and including objective neurophysiological and cognitive measures are required.
Cognitive Candidates for tDCS
Because of its subtle and diffuse effects tDCS is poorly suited to target specific aspects of cognition. It is plausible that prefrontal tDCS could alter processing in numerous addiction related cognitive domains, including risky decision making, cognitive control, response inhibition, sensitivity to reward/punishment, and attentional bias. While a number of addiction studies have documented transient modulation in these domains (Fecteau et al. 2014; Nakamura-Palacios et al. 2016), others have not (T E den Uyl et al. 2015; Klauss et al. 2014). It is noteworthy that a recent large (n = 200) well controlled investigation into the impact of tDCS on selective attention and risky decision making in healthy individuals failed to replicate the prior positive findings of smaller tDCS studies (Russo et al. 2017). While it is possible that a ceiling effect may have contributed to this null finding, as healthy individuals do not have pathological cognitive or neural processes to modify, it does highlight the need for caution when extrapolating the results of the many studies that report evidence of tDCS induced cognitive modulation. Overall, the findings from the broad tDCS cognitive neuroscience literature have provided little to no evidence of reliable or substantial modulation in any cognitive domain. It has, however, highlighted significant inter-individual differences in response to tDCS (Strube et al. 2016; Wiethoff et al. 2014) and it may be that an individualized approach of delivering stimulation specifically to patients who are physiologically responsive to it and show a cognitive phenotype related to abnormalities in the prefrontal cortex could be a way forward. Finally, the impact of repeated stimulation sessions, concurrent addiction therapies, and tDCS protocols requires systematic study, and the potential of focal high definition-tDCS montages (HD-tDCS) that use a greater number of stimulating electrodes to induce more focal and robust neurophysiological effects is worthy of investigation (Hill et al. 2017).
Transcranial Magnetic Stimulation for Addictions
rTMS involves application of a time-varying magnetic field to a target region of the scalp via a stimulating coil. The magnetic field penetrates the skull and induces a focal electrical current which depolarizes underlying cortical neurons. Unlike tDCS, the strength of rTMS pulses are sufficient to induce action potentials. The direction and duration of effects are governed by the intensity, number, properties and pattern of applied stimulation pulses. High-frequency rTMS (>5 Hz) tends to have excitatory effects and is applied with intention of up regulating activity in the targeted brain region, whereas low-frequency rTMS (< 2 Hz) tends to have inhibitory effects and is applied with the intention of down regulating activity in the targeted region (Chen et al. 1997; Siebner et al. 2000) although as with tDCS individual variations in response can be substantial (Maeda et al. 2000). Several types of TMS coils have been developed which differ in the focality and penetrative depth of the stimulation. rTMS for addiction trials have used both figure-of-eight coils, which produce highly focal stimulation in superficial cortex, and H-coils, known as deep-TMS, which are designed to achieve deeper intracranial penetration and stimulate a broader region of tissue extending from the cortical surface through to a more ventral target (Deng et al. 2013).
Using the above mentioned criteria, we found 9 studies that investigate rTMS as addiction intervention (see Table 2). As with tDCS, a majority of rTMS trials (six of nine) have targeted the DLPFC by using figure-of-eight coils. Using the H-coil two studies have additionally tested stimulation of the insular and medial prefrontal cortex. The insula is a critical mediator of decision-making that involves weighing the pleasurable interoceptive effects of substance use and other addictive behaviors against their potential negative consequences (Naqvi and Bechara 2010). The medial prefrontal cortex is involved in a myriad of decision making processes (Euston et al. 2012), and plays a particularly important role in (mal)adaptive responses to stressful and rewarding events and, via connections to the hypothalamic pituitary adrenal axis, ventral tegmental area and the nucleus accumbens, may offer a more direct pathway than the DLPFC to neural circuits linked to motivation control, reward and pleasure. As addiction involves dysfunction in multiple neural circuits the ability to non-invasively modulate both cortical and subcortical brain regions makes rTMS an appealing clinical tool for this indication.
The two rTMS trials for nicotine addiction have stimulated the DLPFC, and both reported positive but short-lived outcomes (Table 2). Amiaz et al. (2009) delivered 10 sessions of high-frequency left DLPFC rTMS followed by a maintenance series of six sessions over the subsequent month. While a prominent placebo effect on self-reported cigarette consumption was noted, relative to sham, active rTMS was associated with fewer number of cigarettes smoked, lower nicotine consumption (verified via urine analysis), and reduced cue-induced craving. Beneficial effects, however, were largely lost at six-month follow up. Trojak et al. (2015) targeted the right DLPFC and delivered 10 sessions of low-frequency rTMS throughout the first two weeks of a six-week course of nicotine replacement therapy. Both active and sham rTMS groups reported reduced craving, active rTMS was associated with greater maintenance of abstinence throughout the program (verified via exhaled carbon monoxide analysis), but beneficial effects were no longer evident at six-week follow up.
The largest study of rTMS for addiction is particularly interesting because it is the only study that targeted multiple brain regions and compared alternative stimulation protocols (Table 2). Dinur-Klein et al. (2014) used the H-coil to stimulate bilateral ventrolateral prefrontal and insular cortices and compared high and low frequency rTMS on craving and cigarette consumption. Additionally, they tested the effect of cue exposure before stimulation. Following 13 sessions administered over three weeks, cue-exposure did not substantially influence outcomes and craving did not differ between the groups, but greatest reduction in number of cigarettes consumed per day (self-report and urine analysis) and highest abstinence rates at end of treatment were observed following high-frequency rTMS.
It is notable that, as with a number of the studies outlined above, a number of rTMS trials for alcohol addiction show a therapeutic response with sham treatment, which highlights the necessity of robust sham conditions and evaluation of participant blinding, and reporting of whether or not concurrent addiction therapies are being undertaken by participants. Höppner et al. (2011) reported that, relative to sham stimulation, 10 sessions of high frequency left DLPFC rTMS increased the attentional blink effect to (i.e. reduced preconscious salience of) alcohol-related pictures, but all participants reported reduced craving for alcohol and improved mood irrespective of stimulation condition. Reduction in the amount of alcohol consumed per day in response to both active and sham stimulation was also described by Ceccanti et al. (2015), though active rTMS was specifically associated with reduced craving. Participants underwent 10 sessions of medial prefrontal cortex H-coil stimulation paired with olfactory drink-of-preference cues. However, the application of only within group analyses (i.e., no direct comparisons between outcomes of active and sham TMS) and high dropout rate during the follow up assessment period limit conclusions that can be drawn from the results.
The only other investigation to encompass medial prefrontal cortex stimulation was conducted by Bolloni et al. (2016) in a cohort of participants with cocaine addiction (Table 2). High frequency bilateral prefrontal deep-TMS was applied three times per week for four weeks and a significant reduction in cocaine use, as indexed by hair toxicology, observed following both active and sham stimulation. When the two groups were analyzed separately, however, persistent reduction in cocaine use three and six months post treatment were significant only for participants who underwent active rTMS.
The single trial of rTMS for methamphetamine addiction is also the only controlled trial to include cognitive outcome measures, albeit largely cognitive measures that are indirectly implicated in addiction decision making (e.g., error monitoring or working memory). Su et al. (2017) delivered five daily sessions of high frequency left DLPFC rTMS as part of a mandatory addiction treatment program. They found a reduction in the intensity of visual drug-cue induced craving in active relative to sham stimulation, and reduction in already low depression severity in both sham and active stimulation, and no differences in anxiety scores in either condition. Across cognitive measures a small improvement in verbal learning and emotional facial recognition following active rTMS was reported, while working memory, problem solving and error monitoring, and visuospatial memory were unchanged.
There is a lack of controlled trials investigating rTMS for behavioral addictions. Two trials have investigated rTMS for bulimia nervosa (as standalone treatment), often conceptualized as one of the numerous presentations of food addiction, and neither found it to be effective beyond sham stimulation. Gay et al. (2016) did not see any beneficial effect on number of binge eating episodes or pre-binge food craving following 10 sessions of active or sham high frequency left DLPFC rTMS. Following 15 sessions of high frequency left DLPFC rTMS, Walpoth et al. (2008) observed significant improvements in binge frequency, obsessive-compulsive and depression symptoms in both the active and sham stimulation conditions.
In summary, the state of evidence for rTMS for addictions is similar to that of tDCS, with a number of promising findings reported alongside numerous negative and inconstant outcomes. The diversity in outcomes likely stems from short treatment courses, variable rTMS protocols and participant characteristics, small sample sizes, and frequent use of subjective and/or short-term outcome measures (Table 2). It is noteworthy that while most rTMS addiction trials have used sham TMS coils, the most robust method for participant blinding, many have observed prominent benefit following sham stimulation. As many studies do not specify the treatment status of participants (see Table 2), apparent placebo response may reflect response to a concurrent treatment regimen. To clarify this issue, future studies should control for and report the treatment status of patients during rTMS treatment and report the efficacy of participant blinding.
Cognitive Candidates for rTMS
As a depolarizing technique that can induce focal or deep stimulation, rTMS is somewhat better placed than tDCS to target specific neural circuits and thereby specific aspects of cognitions. There are a few rTMS studies in addiction that have additionally investigated its effect on different aspects of impulsivity. Impulsivity has been associated with vulnerability for addiction (Allen et al. 1998; Diergaarde et al. 2008). Several studies looked at choice impulsivity and there are indications that rTMS can affect delay discounting in smokers, but the results are ambiguous, and clarification awaits further studies using consistent delay discounting paradigms and examining the impact of rTMS parameters (Bickel et al. 2017; Sheffer et al. 2013; Zack et al. 2016). Moreover, no effects above sham stimulation were found on motor impulsivity (go/no-go task) using a single right DLPFC rTMS session in patients addicted to alcohol (Herremans et al. 2013). A more promising avenue might be to use theta burst TMS that more closely mimics the intrinsic neural oscillations that support cognitive processing (Bickel et al. 2017; Zack et al. 2016). In addition, precision targeting methods such as fMRI guided targeting of cortical regions with strong functional connectivity to subcortical targets of interest have both shown potent cognitive effects (Verbruggen et al. 2010; Wang and Voss 2015). This latter method is a particularly promising avenue to modulate key subcortical nodes within the addiction decision making neurocircuitry, such as the ACC or NA.
Neurofeedback
With neurofeedback, the brain is not directly stimulated as with the previous interventions, but patients learn to modulate their own brain activity through feedback. The brain activity of a patient is monitored and when it shows either the desired or unwanted qualities the patient is informed though visual feedback (e.g., a thermostat that increases when more activity in the desired frequency or brain region) or auditory feedback. Over time the patient will then develop strategies to increase the desired brain activity or decrease the unwanted brain activity. It is therefore a less direct way of neuromodulation that involves training of the participant. Because the two types of neurofeedback that have been used to treat addiction, fMRI and EEG neurofeedback, differ much in the duration they have been used, the number of studies available, the approach (used as stand-alone or add-on treatment), and design, we will use separate criteria for inclusion.
For EEG neurofeedback many studies, case reports and case series have been published but the vast majority is of poor quality: small sample sizes, few training sessions, lack of control condition. We exclusively on studies that meet the following criteria:
-
a.
Minimal sample size of 10 per group
-
b.
10 or more EEG neurofeedback sessions
-
c.
Inclusion of a control condition without neurofeedback
In contrast to EEG neurofeedback the application of fMRI neurofeedback as intervention for addiction is very new. The first studies were published in 2013 and are mostly proof of principle studies with the aim of investigating (1) whether participants can modulate the targeted brain activity (2) and whether this affects craving. No studies to date have investigated the effects of fMRI on drug use; have used sample sizes larger than 15; or used more than 3 fMRI neurofeedback visits. Moreover, only three studies used a control group (Hartwell et al. 2016; Karch et al. 2015; Kirsch et al. 2016). Since the limited total number of studies on the effect of fMRI neurofeedback on craving, we will not use exclusion criteria. However, we will take this lack of criteria with us in the final evaluation of the different techniques in the discussion.
EEG Neurofeedback
The oldest method of neurofeedback is EEG neurofeedback. Electroencephalography (EEG) is a neuroimaging method that records electric activity of the brain through electrodes placed along the scalp. It can measure neural oscillations in specific frequency domains that are associated with functions such as attention or relaxation. With EEG-neurofeedback participants can be trained to amplify or decrease oscillations in certain frequency bands. For instance, in patients with Attention Deficit Hyperactivity Disorder EEG neurofeedback is often used to decrease theta oscillations that are related to relaxation and to amplify beta oscillations associated with concentration (Butnik 2005).
With our search we found only seven studies that investigated EEG neurofeedback as treatment for substance and non-substance related addiction (i.e., used an addiction related outcome measure) (see Table 3).
The first EEG neurofeedback treatment studies for addiction go back to the seventies and focused on training participants to modulate alpha oscillations, which would improve their ability to relax. The underlying idea was that this training could have a therapeutic effect on the addicted individuals either directly or via secondary improvements such as anxiety reduction (Sokhadze et al. 2008). Yet the results on addiction related outcome measures were disappointing (Jones and Holmes 1976; Passini et al. 1977). At the end of the eighties Peniston and Kulkosky popularized the use of EEG neurofeedback as treatment for addiction with a new protocol for alcohol addicted patients that modulated both alpha (8–13 Hz) and theta (4–8 Hz) frequencies in order to bring patients in a relaxed or meditative state (alpha-theta protocol). About ten years later this protocol was adapted by Scott et al. (2005) who expanded alpha-theta training, with beta (16-21 Hz) or sensorimotor rhythm (SMR) (12–15 Hz) training, which aims to improve inhibition. This protocol was thought to be more suitable for patients with other addictions than alcohol, specifically stimulant addiction. The alpha-theta and the alpha-theta augmented with SMR training are now the two main protocols used for treating addiction with EEG neurofeedback (see Table 3).
The Peniston and Kulkosky study (1989) developed the alpha-theta protocol and found more sustained prevention of relapse (defined as not using alcohol for more than 6 contiguous days) in 13-months follow up in alcohol addicted patients who were treated with EEG neurofeedback as add-on treatment compared to patients who only received standard treatment. However, a later study by Lackner et al. (2016) who also used the theta-alpha protocol found no effects on craving or on the 5-month follow up assessment in a group of alcohol addicted in-clinic patients. This same protocol was used in opioid users add-on to psychopharmacological treatment, where they found reduced craving compared to the group that only received medication (Arani et al. 2010).
The combined alpha-theta and SMR training developed by Scott et al. (2005) was first investigated as an add-on treatment for standard rehabilitation program, where they observed longer treatment compliance, and a higher percentage of abstinence (77% of experimental group compared to 44% of control group) at 12 months follow up compared to a control group that received additional counseling (matched in time to neurofeedback sessions). Two more studies used SMR-training in addition to theta-alpha protocol to treat methamphetamine (Rostami and Dehghani-Arani 2015) or opioid (Dehghani-Arani et al. 2013) addiction and found decreased severity of addiction and reductions in craving compared to the treatment as usual (pharmacotherapy) control group. Neither study included a follow up assessment. Finally, the last study looked at a subclinical group of women with binge eating episodes but is interesting to mention in the light of the broader non-substance addiction perspective. They used a different protocol that aims to reduce high beta activity (23–28 Hz) and found reduced frequency of binge eating that remained stable over a 3-month follow up (Schmidt and Martin 2016).
Considering the years that EEG neurofeedback has been studied the low number of studies of reasonable quality is disappointing, which suggests a lack of interest of the field for a well-established efficacy. Even the above discussed studies use a wide variety of outcome assessments which makes the results hard to compare and only one study used a control condition matched in time (Scott et al. 2005). The SMR alpha-theta protocol showed the most promising results for the treatment of addiction but more systematic study is needed to establish for which patients it is most effective, the impact of concurrent therapy, and the optimal protocol.
Cognitive Candidates for EEG Neurofeedback
Two studies (additionally) investigated the effect of mixed SMR theta-alpha EEG neurofeedback on motor-impulsivity in a group with mixed-substance abuse as measured by a cognitive go/no-go task (Keith et al. 2015; Scott et al. 2005). They both found a reduction in commission errors (i.e., responses to no-go trials) in the group that received neurofeedback compared to the control group (with alternative therapy). This suggests the mixed SMR alpha-theta protocol could be specifically effective for treating addiction in high impulsive patients.
fMRI Neurofeedback
Functional Magnetic Resonance Imaging (fMRI) measures increases or decreases of blood oxygenation level dependent (BOLD) response in areas of the brain indicating increased or decreased activity of that area. The main advantage of fMRI over EEG is that it can cover the whole brain (including subcortical areas) and has a much higher spatial resolution. However, preprocessing and analysis of fMRI data used to take much time and thereby made it unsuitable for direct feedback. This changed with the invention of real-time fMRI, where the data are analyzed during collection enabling direct feedback (deCharms 2008). With fMRI neurofeedback it is possible to provide feedback about the activity of one region, a combination of regions or about the functional connectivity between regions. This feedback is mostly visually presented to participants for instance by a thermostat that moves up or down when the targeted activity or connectivity increases or decreases. Therefore, fMRI neurofeedback makes it possible to train people to modulate the activity of specific brain regions or connections.
We found 8 studies that investigated the effects of fMRI neurofeedback on craving (see Table 4).
In total six studies have investigated the effects of fMRI neurofeedback on nicotine addiction (Canterberry et al. 2013; Hanlon et al. 2013; Hartwell et al. 2013, 2016; Kim et al. 2015; Li et al. 2013). Five of these studies showed that modulating activity from ACC with fMRI neurofeedback while watching smoke related pictures was effective in reducing ACC activity and craving (Canterberry et al. 2013; Hanlon et al. 2013; Hartwell et al. 2013, 2016; Li et al. 2013). Yet, when they were instructed to resist craving and received feedback from the (dorso)medial PFC it did not affect brain activity or craving (Hanlon et al. 2013; Hartwell et al. 2016; Li et al. 2013). The fifth study compared feedback from different craving-related regions of interest (ACC, mPFC and OFC) with feedback that additionally gave information about the functional connectivity between posterior and anterior craving-related regions. They found a stronger effect on brain activity and craving, and a correlation between those two, in the condition with additional functional connectivity information (Kim et al. 2015).
The first two studies on fMRI neurofeedback in alcohol addiction (Karch et al. 2015; Kirsch et al. 2016) indicate that patients are capable of reducing craving related activity in the ACC, PFC, insula (Karch et al. 2015), or in the ventral striatum (Kirsch et al. 2016) (see Table 4). In the latter study the researchers found both in the experimental and sham-control group an increase in PFC activity, which was only related to a reduction in the ventral striatum in the experimental group. Although the study of Karch et al. (2015) did scan a control group, they did not include them in the analysis which makes the interpretation of the results difficult.
In sum, at this moment the evidence for fMRI neurofeedback as intervention for addiction is still scarce and we will need larger controlled trials to establish efficacy on drug use and long-term effects.
Cognitive Candidates for fMRI Neurofeedback
Since fMRI is the most commonly used tool to investigate the biological basis of neurocognitive processes related to addiction other than craving, fMRI neurofeedback could be very suitable to target these processes such as reward processing or cognitive control. Hopefully future research will explore these promising avenues.
Invasive Brain Stimulation: Deep Brain Stimulation (DBS)
In addition to the above mentioned non-invasive techniques, deep brain stimulation (DBS) offers a more direct way for stimulating and modulating the brain. Powered by an implanted pulse generator, DBS provides deeper located brain areas with a continuous stimulation via surgically implanted microelectrodes. The implanted pulse generator, which is located in the pectoral area, can be manually switched on and off from outside the body and furthermore allows for modulation of stimulation frequency and intensity. Originally, DBS was introduced in the 80’s as treatment for intractable movement disorders. However, in the last 15 years it has been successfully applied in psychiatric disorders such as treatment-refractory depression and treatment-refractory obsessive-compulsive disorder (OCD). When studies reported unintended alleviation of comorbid alcohol (Kuhn et al. 2007) and nicotine (Kuhn et al. 2009) dependences following nucleus accumbens (NAc) DBS and reduced pathological gambling (Ardouin et al. 2006) in patients with Parkinson’s disease, DBS gained interest as a tool for addiction treatment.
Since then, several studies have examined the possibility of DBS as a therapeutic tool in addiction treatment. In 2012, Luigjes et al. reviewed possible brain targets for DBS in treatment-refractory addiction based on previously conducted clinical and animal studies. Human studies mainly focused on NAc and subthalamic nucleus (STN) as these areas have been regularly used in other refractory psychiatric disorders. Luigjes et al. (2012) suggested the NAc as most promising target for DBS therapy since both animal and human studies with NAc stimulation showed reduction or cessation of drug intake and long-term abstinence without severe side effects.
The effectiveness of NAc stimulation may be, in part, explained by its central position in brain circuits implicated in motivation and inhibitory control. As a result of repeated drug use the brain circuit implicated in motivational drive could become hyperactive in response to the substance. At the same time, diminished activation of prefrontal areas implicated in regulation of inhibitory processes such as impulse control and decision-making could result in a deficient inhibitory system (Volkow et al. 2013). Combined, these disturbances could result in a heightened drive to obtain and use drugs while it becomes harder to suppress this persistent drive as a result of a deficient inhibitory system.
All studies that investigated the efficacy of DBS for addiction are case reports or case series. To the best of our knowledge, no larger controlled trials have been published. Therefore, we will discuss all the currently available human DBS (case) studies. Currently eight studies in a total of 11 patients have specifically examined the effects of DBS on addiction related behavior (see Table 5). Six out of these eight studies examined unique patients with a treatment resistant course of alcohol, heroin, or cocaine addiction and all but one used the NAc as stimulation target.
DBS Studies
In two studies six patients were treated with bilateral high-frequency NAc DBS for severe alcohol dependence (Table 5). All six patients showed marked reduction of alcohol craving and intake. Two patients, examined in the study of Müller et al. (2016), even remained abstinent during a follow up period of seven years. The patient examined in the study of Kuhn et al. (2011) showed reduction of craving and ceased drinking one year after initial treatment. Additionally, complemented electroencephalography (EEG) measurements in this patient revealed stronger error related activity in the anterior cingulate cortex (ACC) compared to the situation prior to DBS stimulation. This may perhaps indicate that the patient regained cognitive control as result of NAc DBS.
Three studies examined the effect of bilateral high-frequency NAc stimulation in four patients with a chronic treatment resistant course of heroin dependence (Table 5). In the case series of Kuhn et al. (2014) DBS reduced craving and induced heroin abstinence in two treatment resistant patients. Although DBS induced heroin abstinence, both patients reported occasional use of other psychotropic substances such as amphetamine and alcohol. According to the two patients, comorbid substance use resulted from boredom and incapability to cope with occupational strains rather than craving. In the two remaining case reports, one patient remained abstinent and free of craving for the complete follow up period of six years, even when electrodes were first switched-off and explanted after three years (Zhou et al. 2011). The other patient treated with NAc DBS for his heroin dependence reduced his heroin consumption over a period of months and remained abstinent during the 1-year follow up with the exception of a two-week relapse (Valencia-Alfonso et al. 2012).
Finally, a 36-year-old patient with severe refractory cocaine dependence was treated with high-frequency DBS (Table 5). Microelectrodes were implanted in the posterior part of the medial ACC and bed nucleus of stria terminalis. Initiation of DBS after surgery resulted in a considerable reduction of craving and cocaine consumption which was maintained more than 2.5 years post-surgery (Gonçalves-Ferreira et al. 2016).
Although results from the above-mentioned case studies and case reports may seem promising, as of today no double blind controlled DBS trials in addiction are available. It is possible that the clinical application of DBS is regarded as too costly and invasive for the treatment of addiction. Recruitment of sufficiently motivated patients for controlled studies proves difficult (Luigjes et al. 2015). At the same time, recruitment difficulties could be a result of the absence of strong evidence for the effectiveness of DBS and the suitability as technique for addiction, its proven effectiveness in other neuropsychiatric disorders (Denys et al. 2010). Because of this, DBS addiction research seems to have reached an impasse. Therefore, it remains difficult to draw conclusions in terms of effectiveness.
Cognitive Candidates for DBS
The lack of controlled double-blind studies shows that DBS addiction research is still in an early stage and the effects of DBS on addiction related cognitive processes remain speculative. Nevertheless, animal and case studies have provided evidence that NAc DBS can modulate impulsivity. Since impulsivity plays an important role in addiction it is a possible future cognitive target candidate (Sesia et al. 2008, 2010). Yet, several human and animal studies indicate that NAc stimulation can modulate impulsivity in both directions. Whether impulsivity is increased or decreased depends on multiple factors including the exact region of stimulation within the NAc (i.e. NAc core or shell), baseline impulsivity levels in animals, stimulus intensity and on which behavioral paradigm was used (Schippers et al. 2017; Sesia et al. 2008, 2010). Additionally, animal studies have observed that DBS differentially affects state impulsivity and trait impulsivity as the effect of DBS could sometimes have a different direction on these two forms of impulsivity (Schippers et al. 2017; Sesia et al. 2010). These studies show that controlled modulation of impulsivity with NAc DBS is very complex and caution is warranted since increased impulsivity may lead to relapse (Dalley et al. 2011). Indeed, such an increase in impulsivity was observed in two refractory OCD patients who were treated with NAc DBS (Luigjes et al. 2011). Therefore, more research is needed to understand how DBS affects impulsivity, and impulsive behavior should be carefully regarded during patient screening and selection in DBS treatment (Luigjes et al. 2011).
Candidate Targets for Neuromodulation
Several neurocognitive aspects of addiction can also be found in psychiatric disorders more commonly treated with neuromodulation. The effects of neuromodulation on these shared neurocognitive aspects could reveal potential brain modulation targets for addiction. As an example of such neurocognitive overlap, aberrant reward processing is central to addiction but is also found in OCD. Blunted monetary reward anticipation signals in the ventral striatum have been demonstrated in addiction to alcohol, nicotine and cannabis (Bühler et al. 2010; van Hell et al. 2010; Wrase et al. 2007), and in a behavioral addiction like pathological gambling (de de Greck et al. 2010; Reuter et al. 2005). Similarly, patients with OCD display blunted monetary reward anticipation activity in the ventral striatum compared to healthy controls (Figee et al. 2011, 2013). Effective DBS of the ventral anterior limb of the internal capsule (vALIC) in OCD patients is able to normalize these anticipatory reward responses in the ventral striatum (Figee et al. 2013). Of interest, effective DBS for major depression, a disorder that is also associated with ventral striatal reward dysfunction (Pizzagalli et al. 2009), appears to be dependent on stimulation of comparable ventral anterior internal capsule fibers (Lujan et al. 2012). Together, these findings in various disorders of reward dysfunction suggest that DBS targeted at the vALIC may also be effective in addiction via restoration of healthy ventral striatal reward processing. Indeed, vALIC DBS has shown preliminary effectiveness in drug addiction (Valencia-Alfonso et al. 2012).
Addiction and OCD also share a more general dysregulation of the ventral striatum-prefrontal network, in particular frontostriatal functional hyperconnectivity. Studies in OCD demonstrate that this frontostriatal connectivity can be normalized with DBS of the vALIC (Bahramisharif et al. 2016; Figee et al. 2013; Smolders et al. 2013), but also with rTMS of the mPFC (Dunlop et al. 2016). Preliminary evidence suggests that the vALIC and mPFC are also effective targets for DBS and TMS in addiction based on a comparable down-regulation of frontostriatal hyperconnectivity (De Ridder et al. 2011; Figee et al. 2013) Of interest, DBS resulted in attenuated craving for heroin in a patient with heroin addiction when exactly those contact points on the electrode were activated at which the lowest ventral striatal-prefrontal connectivity in response to drug-related pictures was measured (Valencia-Alfonso et al. 2012). Therefore, down-regulation of ventral frontostriatal connectivity could be examined as a personalized outcome for DBS and rTMS in the treatment of addiction.
Another neurocognitive aspect underlying addiction is an overreliance on negative-reinforcement in anti-reward brain systems. Important structures implicated in these anti-reward systems are the extended amygdala (bed nucleus stria terminalis, BNST) and the lateral habenula (Root et al. 2014), which are associated with aversive or stress-like states. Adaptations of these systems may persist during and beyond drug abstinence, creating a condition of chronic dysphoria and increasing the likelihood of relapse in a compulsive attempt to self-medicate this unwanted condition (Vollstädt-Klein et al. 2010). Negative reinforcement in addiction may be normalized with DBS targeted at brain anti-reward systems such as the BNST or lateral habenula. BNST DBS was reported effective in a patient with refractory cocaine dependence (Gonçalves-Ferreira et al. 2016). Habenula stimulation inhibited cocaine seeking in rats (Friedman et al. 2011).
In addition, medial prefrontal-amygdala circuits involved in fear conditioning and extinction have been implicated in persistent drug-seeking behavior and relapses (Crunelle et al. 2015; Peters et al. 2009). Interestingly, ventral striatal (VS) high frequency DBS in rats, comparable to the settings often used in humans, impaired extinction training of morphine seeking, whereas low frequency dorsal VS DBS strengthened morphine extinction memory (Martínez-Rivera et al. 2016). DBS in this study also modulated activity (c-fos expression) in areas mediating extinction such as the mPFC and amygdala. Thus, low frequency DBS of the dorsal VS might be beneficial for preventing relapses to drug addiction by activating the mPFC-amygdala circuit involved in extinction learning. In line with the potential of mPFC modulation for extinction of addiction-associated negative reinforcement, rTMS of the mPFC was able to reduce craving in alcohol addiction (Ceccanti et al. 2015) and resulted in persistent reduction in cocaine use (Bolloni et al. 2016).
The insula may be another promising neuromodulation candidate for extinction in the prevention of drug relapse. The insula is activated during exposure to drug-associated cues in addicted individuals (Garavan 2010) and its inactivation has been associated with decreased relapse rates of nicotine addiction (Gaznick et al. 2014; Naqvi et al. 2007). Inhibition of the anterior insular cortex may facilitate extinction of drug seeking after abstinence via modulation of projections to the central amygdala, as was shown in a rat model (Venniro et al. 2017). Although selective neuromodulatory inhibition of the anterior insula has not yet been tried in people with addictive disorders, the insula was recently selectively targeted in healthy individuals with rTMS using an H-coil (Malik et al. 2017). Of interest, low frequency (presumably inhibitory) rTMS of the insula decreased dopamine levels in the substantia nigra and striatum, suggesting that inhibitory neuromodulation of the insula may also influence reward processing.
Finally, it is relevant to note that DBS of the STN is often beneficial for addictive behaviors related to poor impulse control in Parkinson patients. In the majority of cohort studies and case studies, STN DBS diminishes the prevalence of impulse control disorders, such as pathological gambling, shopping, binge eating or dependency towards their dopamine medication, relative to the preoperative situation (Merola et al. 2017). Nevertheless, a reduction of dopaminergic medication after DBS surgery may partly contribute to this improvement of impulse control disorders. Moreover, there have also been measures of impulsivity, such as response inhibition and delay discounting that deteriorated with STN DBS in Parkinson patients (Georgiev et al. 2016). In rats, STN DBS decreases the motivation for cocaine (Rouaud et al. 2010). In parallel to the beneficial effect of STN lesions for addiction (Baunez et al. 2005), STN DBS may be decrease motivation for drug rewards via inhibition of STN excitatory input on the ventral midbrain, resulting in attenuated dopaminergic activity.
Discussion
At the moment, little evidence supports the hope that neuromodulation will soon become a new effective intervention for addiction. This may be due to a lack of well-powered systematic and controlled studies. In addition, the reviewed studies indicate that it may too simplistic to assume that neuromodulation targets specific brain regions. Non-invasive neurostimulation and EEG neurofeedback are targeted at broader brain regions or networks, as well as DBS, where specific regions are stimulated and distant brain regions are critically involved in the clinical effects. Moreover, due to the heterogeneity of addiction, neurobiological knowledge may not be easily translated into treatment targets. In sum, it is important to realize the complexity of both the neurobiology of addiction and the effects of neuromodulation when applying neuromodulation. And one way forward could be to use study designs that integrate and test more complex models of neuromodulation.
Because of the different number and quality of studies available for each neuromodulation technique we used separate inclusion criteria. This means that we cannot directly compare the efficacy of these studies and therefore we will discuss them separately with respect to the stage of development they are in. The studies with DBS and fMRI neurofeedback are either pilot or proof-of-principle studies that do not warrant any conclusions about the efficacy as addiction interventions. This is not surprising since these interventions as indication for addiction are still new, require a high degree of expertise and are expensive. As Table 4 shows, the fMRI neurofeedback studies are all published in the last five years and have developed over time in terms of quality and samples size, therefore it is to be expected that larger controlled trials investigating the effect on drug use will follow. With DBS, difficulties with the inclusion of patients have hampered larger controlled DBS studies, while such trials have been published for the treatment of depression and obsessive-compulsive disorder. It is unknown whether future developments in the field will overcome these difficulties.
With rTMS and tDCS, most studies have focused on immediate effects of craving. Yet there are a few studies that looked at (immediate and longer-term) effects on drug use, with promising results indicating the potential of these techniques for treating addiction. In this regard, the current state of research is not dissimilar to the early years of experimental rTMS for major depression, which eventually has become an effective treatment. Whether rTMS will ultimately be an effective treatment for addiction as well, awaits larger trials delivering longer treatment courses, incorporating objective and long-term outcome assessments, and systematic investigation of stimulation protocols.
On the other hand, EEG neurofeedback has been investigated for decades and although one of the first studies included a sham feedback control condition (Jones and Holmes 1976), very few studies afterwards have used an appropriate control with either sham-feedback or where at least the time spend on the neurofeedback sessions was matched with another activity. This makes it difficult to draw conclusions about the specific effect of EEG neurofeedback, since any effects might be the result of the extra time and care patients received for the neurofeedback sessions. In one study that did include such a control condition, the control condition was not used as comparison in the final analysis (Schmidt and Martin 2016). Fortunately, there are two exceptions with control condition (time-matched activity) (Keith et al. 2015; Scott et al. 2005) suggesting that the combination of SMR and alpha-theta neurofeedback can have a positive effect on impulsivity, and on the period of time spend in rehabilitation. However, after decades of investigating EEG neurofeedback the lack of well-designed studies is worrisome and raises the question whether this reflects the lack of potential of EEG neurofeedback to treat addiction.
Until now all fMRI neurofeedback studies and most rTMS/tDCS studies have focused on short-term outcome assessments. Yet, in order to apply these neuromodulation techniques as intervention for addiction a crucial question is whether it is possible to bring about lasting changes in well-established addictive behavior. To answer this question, we should not only assess long-term outcomes but also investigate the number of treatment sessions required for these lasting behavioral changes. In most studies short treatment courses were used: fMRI neurofeedback studies (max 3), tDCS (max 10), rTMS (max 16), only in EEG neurofeedback four studies used 30 sessions or more. This might have contributed to the lack of long-term effects found by several studies: Da Silva et al. (2013) with 5 sessions, Amiaz et al. (2009) with 16 sessions, Trojak et al. (2015) with 10 sessions and Lackner (2016) with 12 sessions. It is likely that in order to change neural programs and their associated behavior, extensive practice is needed using more sessions. Therefore, future research in this area should address the dose of neuromodulation requires to achieve persistent long-term reduction in addiction severity.
Moreover, the second main concern of the reviewed studies is that many of them are underpowered. Two fMRI neurofeedback studies (Canterberry et al. 2013; Kim et al. 2015), five rTMS studies (Bolloni et al. 2016; Ceccanti et al. 2015; Dinur-Klein et al. 2014; Höppner et al. 2011; Walpoth et al. 2008) and three tDCS studies (Conti and Nakamura-Palacios 2014; da Silva et al. 2013; Fecteau et al. 2014) used less than 10 participants per condition. Four DBS only case reports or series have been published. This warrants caution with the interpretation of these results, which have a higher change of type II error and inflated effect sizes. However, in all fields except for EEG neurofeedback, increasing the quality of studies for our review by excluding those with less than 10 participants per condition would not have weighed up against the low number of studies left with (0 dB, 6 fMRI neurofeedback, 4 tDCS and 5 TMS). Fortunately in recent years sample sizes are increasing (e.g., Gay et al. 2016; Hartwell et al. 2016; Ljubisavljevic et al. 2016), and in 2017 the largest tDCS trial in addiction was published. In this study, 91 patients were randomized into three groups with the active intervention group receiving a single session tDCS to boost a cognitive bias modification training. No effects of tDCS were found on craving, approach bias or abstinence after three months, but there was a trend towards lower relapse in the tDCS condition after one year (den Uyl et al. 2017). In sum, while there is still a lack of well-powered studies the field seems to be progressing in the right direction and future studies should aim for larger samples sizes.
Neurofeedback, rTMS and tDCS have very few side effects, and are transient; therefore, they are considered safe interventions (Priori et al. 2009). DBS is invasive and side effects such as transient hypomanic episodes have been reported. Additionally, there is a small risk of hemorrhage, infection or headaches. Yet, studies of DBS targeted at the ventral striatal area for other psychiatric disorders have proven to be safe and modulate neurocognitive dysfunctions that are also relevant for addiction (Luigjes et al. 2013). For neuromodulation and non-invasive neurostimulation, future research will have to investigate the number of sessions needed for an optimal effect, and whether patients will benefit from booster sessions after treatment. DBS gives chronic stimulation; however, one case report in a patient treated for opioid addiction showed that the patient remained abstinent even with cessation of stimulation (Zhou et al. 2011).
One question that remains to be investigated is whether neuromodulation techniques can function as stand-alone treatments or whether they are more effective as an add-on treatment. In the majority of EEG neurofeedback studies, the treatment is provided as add-on to either pharmacological treatment or psychotherapy with the rationale that EEG neurofeedback may augment the effects of treatment as usual. Moreover, two tDCS studies used concurrent therapy (de Silva et al. 2003; Klauss et al. 2014) and one rTMS study (Trojak et al. 2015) used additional nicotine replacement treatment. Kuhn et al. (2014) proposed that additional behavioral therapy may be helpful for patients to learn to cope with stress and boredom after DBS treatment which are often triggers for relapse. The combination of DBS and cognitive behavioral therapy has been successful in the treatment of obsessive-compulsive disorder (Mantione et al. 2014). Since addiction is a disorder that affects many aspects of a patient’s life it is likely that additional cognitive behavioral treatment or psychosocial support can play an important role in the success of neuromodulation interventions especially for the most severely affected patients. Alternatively, neuromodulation could enhance the success of add-on treatment. rTMS and DBS have substantial efficacy in mood disorders (Kisely et al. 2018; Perera et al. 2016), and beneficial effects on mood in addiction could enhance motivation for change and ability to engage in complementary therapies. Moreover, combining neuromodulation with other treatments may result in beneficial synergistic effects at cognitive and neural levels. For example, combining neuromodulation and cognitive training has been shown to be beneficial for major depression and Alzheimer’s disease, when the training involves exercising a cognitive skill required for recovery. For patients with addictions, using neuromodulation techniques to induce plasticity whilst exercising key cognitive skill such as response inhibition, extinction learning, or cognitive re-framing, may have greater potential to modify neural networks and cognitive processes driving addictions, and potentially induce greater long-term effects (Rabey et al. 2013; Segrave et al. 2014).
Future Perspective
A way forward in the application of neuromodulation for addiction might be to tailor these interventions to individual patients or subgroups of patients based on cognitive or neural profiles. First, this can be done by targeting specific cognitive candidates that are likely to map more closely to neural abnormalities than general addiction outcomes as craving or drug use. For instance, an overreliance on negative-reinforcement may indicate modulation of anti-reward brain network, whereas high impulsive patients may benefit more from targeting regions involved in cognitive control. Moreover, profiling based on neural functioning might be another way to investigate for which patients’ specific types of neuromodulation are most effective. Although few studies in the addiction field investigated or found effects on specific cognitive processes and none used profiling, studies in other fields show reason to be optimistic about this approach. A study that focused on rTMS for depression found that based on MRI resting state scans depressed patients can be divided in four subtypes related to specific symptom profiles that predict the response on rTMS treatment (Drysdale et al. 2016). Additionally, for neurofeedback approaches there are indications that certain participants are better able to modulate their brain activity. Better prediction of which patients will be successful can lead to better targeted neurofeedback treatment which can be especially important for the more expensive fMRI neurofeedback. A possible cheap method of predicting patients’ success is selecting patients that are able to modulate their temperature with biofeedback (Hartwell et al. 2013)
Moreover, there are encouraging developments in other psychiatric indications for neuromodulation that show how the interaction between neurobiological science and the application of neuromodulation can mutually advance each other. For instance, a German research group found a more optimal new target for the treatment of DBS in depression, the medial forebrain bundle, based on translational neurobiological research (Bewernick et al. 2017; Coenen et al. 2011). A different group found that scans informing about structural brain connectivity could help predict the optimal DBS target for individual patients with depression (Riva-Posse et al. 2018). These studies in other psychiatric disorders show a possible way forward to improve future application of neuromodulation in addiction treatment.
References
Allen, T. J., Moeller, F. G., Rhoades, H. M., & Cherek, D. R. (1998). Impulsivity and history of drug dependence. Drug and Alcohol Dependence, 50(2), 137–145.
Amiaz, R., Levy, D., Vainiger, D., Grunhaus, L., & Zangen, A. (2009). Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction, 104(4), 653–660.
Arani, F. D., Rostami, R., & Nostratabadi, M. (2010). Effectiveness of neurofeedback training as a treatment for opioid-dependent patients. Clinical EEG and Neuroscience, 41(3), 170–177. https://doi.org/10.1177/155005941004100313.
Ardouin, C., Voon, V., Worbe, Y., Abouazar, N., Czernecki, V., Hosseini, H., et al. (2006). Pathological gambling in Parkinson’s disease improves on chronic subthalamic nucleus stimulation. Movement Disorders: Official Journal of the Movement Disorder Society, 21(11), 1941–1946. https://doi.org/10.1002/mds.21098.
Bahramisharif, A., Mazaheri, A., Levar, N., Richard Schuurman, P., Figee, M., & Denys, D. (2016). Deep brain stimulation diminishes cross-frequency coupling in obsessive-compulsive disorder. Biological Psychiatry, 80(7), e57–e58. https://doi.org/10.1016/j.biopsych.2015.05.021.
Banz, B. C., Yip, S. W., Yau, Y. H. C., & Potenza, M. N. (2016). Behavioral addictions in addiction medicine: From mechanisms to practical considerations. Progress in Brain Research, 223, 79–6123. https://doi.org/10.1016/bs.pbr.2015.08.003.
Batista, E. K., Klauss, J., Fregni, F., Nitsche, M. A., & Nakamura-Palacios, E. M. (2015). A randomized placebo-controlled trial of targeted prefrontal cortex modulation with bilateral tDCS in patients with crack-cocaine dependence. International Journal of Neuropsychopharmacology, 18(12), pyv066. https://doi.org/10.1093/ijnp/pyv066.
Batsikadze, G., Moliadze, V., Paulus, W., Kuo, M. F., & Nitsche, M. A. (2013). Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. Journal of Physiology, 591(7), 1987–2000. https://doi.org/10.1113/jphysiol.2012.249730.
Baunez, C., Dias, C., Cador, M., & Amalric, M. (2005). The subthalamic nucleus exerts opposite control on cocaine and “natural” rewards. Nature Neuroscience, 8(4), 484–489. https://doi.org/10.1038/nn1429.
Bewernick, B. H., Kayser, S., Gippert, S. M., Switala, C., Coenen, V. A., & Schlaepfer, T. E. (2017). Deep brain stimulation to the medial forebrain bundle for depression- long-term outcomes and a novel data analysis strategy. Brain Stimulation, 10(3), 664–671. https://doi.org/10.1016/j.brs.2017.01.581.
Bickel, W., Snider, S., Hanlon, C., & Stein, J. (2017). Continuous theta burst TMS as a tool to change decision-making in smokers. Brain Stimulation, 10(2), 487. https://doi.org/10.1016/j.brs.2017.01.426.
Boggio, P. S., Liguori, P., Sultani, N., Rezende, L., Fecteau, S., & Fregni, F. (2009). Cumulative priming effects of cortical stimulation on smoking cue-induced craving. Neuroscience Letters, 463(1), 82–86.
Bolloni, C., Panella, R., Pedetti, M., Frascella, A. G., Gambelunghe, C., Piccoli, T., et al. (2016). Bilateral transcranial magnetic stimulation of the prefrontal cortex reduces cocaine intake: A pilot study. Frontiers in Psychiatry, 7.
Bühler, M., Vollstädt-Klein, S., Kobiella, A., Budde, H., Reed, L. J., Braus, D. F., et al. (2010). Nicotine dependence is characterized by disordered reward processing in a network driving motivation. Biological Psychiatry, 67(8), 745–752. https://doi.org/10.1016/j.biopsych.2009.10.029.
Butnik, S. M. (2005). Neurofeedback in adolescents and adults with attention deficit hyperactivity disorder. Journal of Clinical Psychology, 61(5), 621–625. https://doi.org/10.1002/jclp.20124.
Canterberry, M., Hanlon, C. A., Hartwell, K. J., Li, X., Owens, M., LeMatty, T., et al. (2013). Sustained reduction of nicotine craving with real-time neurofeedback: Exploring the role of severity of dependence. Nicotine & Tobacco Research, 15(12), 2120–2124. https://doi.org/10.1093/ntr/ntt122.
Ceccanti, M., Inghilleri, M., Attilia, M. L., Raccah, R., Fiore, M., Zangen, A., & Ceccanti, M. (2015). Deep TMS on alcoholics: Effects on cortisolemia and dopamine pathway modulation. A pilot study. Canadian Journal of Physiology and Pharmacology, 93(4), 283–290.
Chen, R., Classen, J., Gerloff, C., Celnik, P., Wassermann, E. M., Hallett, M., & Cohen, L. G. (1997). Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology, 48(5), 1398–1403.
Coenen, V. A., Schlaepfer, T. E., Maedler, B., & Panksepp, J. (2011). Cross-species affective functions of the medial forebrain bundle—Implications for the treatment of affective pain and depression in humans. Neuroscience & Biobehavioral Reviews, 35(9), 1971–1981. https://doi.org/10.1016/j.neubiorev.2010.12.009.
Coles, A. S., Kozak, K., & George, T. P. (2018). A review of brain stimulation methods to treat substance use disorders. The American Journal on Addictions, 27(2), 71–91. https://doi.org/10.1111/ajad.12674.
Conti, C. L., & Nakamura-Palacios, E. M. (2014). Bilateral transcranial direct current stimulation over dorsolateral prefrontal cortex changes the drug-cued reactivity in the anterior cingulate cortex of crack-cocaine addicts. Brain Stimulation, 7(1), 130–132.
Crunelle, C. L., Kaag, A. M., van den Munkhof, H. E., Reneman, L., Homberg, J. R., Sabbe, B., et al. (2015). Dysfunctional amygdala activation and connectivity with the prefrontal cortex in current cocaine users. Human Brain Mapping, 36(10), 4222–4230. https://doi.org/10.1002/hbm.22913.
da Silva, M. C., Conti, C. L., Klauss, J., Alves, L. G., do Nascimento Cavalcante, H. M., Fregni, F., et al. (2013). Behavioral effects of transcranial direct current stimulation (tDCS) induced dorsolateral prefrontal cortex plasticity in alcohol dependence. Journal of Physiology-Paris, 107(6), 493–502.
Dalley, J. W., Everitt, B. J., & Robbins, T. W. (2011). Impulsivity, compulsivity, and top-down cognitive control. Neuron, 69(4), 680–694. https://doi.org/10.1016/j.neuron.2011.01.020.
de Greck, M., Enzi, B., Prösch, U., Gantman, A., Tempelmann, C., & Northoff, G. (2010). Decreased neuronal activity in reward circuitry of pathological gamblers during processing of personal relevant stimuli. Human Brain Mapping, 31(11), 1802–1812. https://doi.org/10.1002/hbm.20981.
De Ridder, D., Vanneste, S., Kovacs, S., Sunaert, S., & Dom, G. (2011). Transient alcohol craving suppression by rTMS of dorsal anterior cingulate: An fMRI and LORETA EEG study. Neuroscience Letters, 496(1), 5–10. https://doi.org/10.1016/j.neulet.2011.03.074.
de Silva, P., Menzies, R. G., & Shafran, R. (2003). Spontaneous decay of compulsive urges: The case of covert compulsions. Behaviour Research and Therapy, 41(2), 129–137.
deCharms, R. C. (2008). Applications of real-time fMRI. Nature Reviews. Neuroscience, 9, 720–729. https://doi.org/10.1038/nrn2414.
Dehghani-Arani, F., Rostami, R., & Nadali, H. (2013). Neurofeedback training for opiate addiction: Improvement of mental health and craving. Applied Psychophysiology and Biofeedback, 38(2), 133–141. https://doi.org/10.1007/s10484-013-9218-5.
den Uyl, T. E., Gladwin, T. E., & Wiers, R. W. (2015). Transcranial direct current stimulation, implicit alcohol associations and craving. Biological Psychology, 105, 37–42. https://doi.org/10.1016/j.biopsycho.2014.12.004.
den Uyl, T. E., Gladwin, T. E., Rinck, M., Lindenmeyer, J., & Wiers, R. W. (2017). A clinical trial with combined transcranial direct current stimulation and alcohol approach bias retraining. Addiction Biology, 22(6), 1632–1640. https://doi.org/10.1111/adb.12463.
Deng, Z.-D., Lisanby, S. H., & Peterchev, A. V. (2013). Electric field depth–focality tradeoff in transcranial magnetic stimulation: Simulation comparison of 50 coil designs. Brain Stimulation, 6(1), 1–13. https://doi.org/10.1016/j.brs.2012.02.005.
Denys, D., Mantione, M., Figee, M., van den Munckhof, P., Koerselman, F., Westenberg, H., et al. (2010). Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Archives of General Psychiatry, 67(10), 1061–1068. https://doi.org/10.1001/archgenpsychiatry.2010.122.
Diergaarde, L., Pattij, T., Poortvliet, I., Hogenboom, F., de Vries, W., Schoffelmeer, A. N. M., & De Vries, T. J. (2008). Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biological Psychiatry, 63(3), 301–308. https://doi.org/10.1016/j.biopsych.2007.07.011.
Dinur-Klein, L., Dannon, P., Hadar, A., Rosenberg, O., Roth, Y., Kotler, M., & Zangen, A. (2014). Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: A prospective, randomized controlled trial. Biological Psychiatry, 76(9), 742–749. https://doi.org/10.1016/j.biopsych.2014.05.020.
Drysdale, A. T., Grosenick, L., Downar, J., Dunlop, K., Mansouri, F., Meng, Y., et al. (2016). Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nature Medicine, 23(1), 28–38. https://doi.org/10.1038/nm.4246.
Dunlop, K., Woodside, B., Olmsted, M., Colton, P., Giacobbe, P., & Downar, J. (2016). Reductions in Cortico-striatal Hyperconnectivity accompany successful treatment of obsessive-compulsive disorder with dorsomedial prefrontal rTMS. Neuropsychopharmacology, 41(5), 1395–1403. https://doi.org/10.1038/npp.2015.292.
Euston, D. R., Gruber, A. J., & McNaughton, B. L. (2012). The role of medial prefrontal cortex in memory and decision making. Neuron, 76(6), 1057–1070. https://doi.org/10.1016/j.neuron.2012.12.002.
Fecteau, S., Agosta, S., Hone-Blanchet, A., Fregni, F., Boggio, P., Ciraulo, D., & Pascual-Leone, A. (2014). Modulation of smoking and decision-making behaviors with transcranial direct current stimulation in tobacco smokers: A preliminary study. Drug and Alcohol Dependence, 140, 78–84.
Figee, M., Vink, M., de Geus, F., Vulink, N., Veltman, D. J., Westenberg, H., & Denys, D. (2011). Dysfunctional reward circuitry in obsessive-compulsive disorder. Biological Psychiatry, 69(9), 867–874. https://doi.org/10.1016/j.biopsych.2010.12.003.
Figee, M., Luigjes, J., Smolders, R., Valencia-Alfonso, C.-E., van Wingen, G., de Kwaasteniet, B., et al. (2013). Deep brain stimulation restores frontostriatal network activity in obsessive-compulsive disorder. Nature Neuroscience, 16(4), 386–387. https://doi.org/10.1038/nn.3344.
Fineberg, N. A., Potenza, M. N., Chamberlain, S. R., Berlin, H. A., Menzies, L., Bechara, A., et al. (2010). Probing compulsive and impulsive behaviors, from animal models to endophenotypes: A narrative review. Neuropsychopharmacology: Official Publication of the American College of. Neuropsychopharmacology, 35(3), 591–604. https://doi.org/10.1038/npp.2009.185.
Friedman, A., Lax, E., Dikshtein, Y., Abraham, L., Flaumenhaft, Y., Sudai, E., et al. (2011). Electrical stimulation of the lateral habenula produces an inhibitory effect on sucrose self-administration. Neuropharmacology, 60(2–3), 381–387. https://doi.org/10.1016/j.neuropharm.2010.10.006.
Garavan, H. (2010). Insula and drug cravings. Brain Structure and Function, 214(5–6), 593–601. https://doi.org/10.1007/s00429-010-0259-8.
Gay, A., Jaussent, I., Sigaud, T., Billard, S., Attal, J., Seneque, M., et al. (2016). A lack of clinical effect of high-frequency rTMS to dorsolateral prefrontal cortex on bulimic symptoms: A randomised, double-blind trial. European Eating Disorders Review, 24(6), 474–481.
Gaznick, N., Bechara, A., & Tranel, D. (2014). Hemispheric side of damage influences sex-related differences in smoking cessation in neurological patients. Journal of Clinical and Experimental Neuropsychology, 36(5), 551–558. https://doi.org/10.1080/13803395.2014.915012.
George, O., & Koob, G. F. (2010). Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neuroscience & Biobehavioral Reviews, 35(2), 232–247. https://doi.org/10.1016/j.neubiorev.2010.05.002.
Georgiev, D., Dirnberger, G., Wilkinson, L., Limousin, P., & Jahanshahi, M. (2016). Parkinson’s disease on a probabilistic go/NoGo task deep brain stimulation of the subthalamic nucleus only interferes with withholding of the most prepotent responses. Experimental Brain Research, 234(4), 1133–1143. https://doi.org/10.1007/s00221-015-4531-2.
Gonçalves-Ferreira, A., Do Couto, F. S., Rainha Campos, A., Lucas Neto, L. P., Gonçalves-Ferreira, D., & Teixeira, J. (2016). Deep brain stimulation for refractory cocaine dependence. Biological Psychiatry, 79(11), e87–e89. https://doi.org/10.1016/j.biopsych.2015.06.023.
Hanlon, C. A., Hartwell, K. J., Canterberry, M., Li, X., Owens, M., Lematty, T., et al. (2013). Reduction of cue-induced craving through realtime neurofeedback in nicotine users: The role of region of interest selection and multiple visits. Psychiatry Research, 213(1), 79–81. https://doi.org/10.1016/j.pscychresns.2013.03.003.
Hartwell, K. J., Prisciandaro, J. J., Borckardt, J., Li, X., George, M. S., & Brady, K. T. (2013). Real-time fMRI in the treatment of nicotine dependence: A conceptual review and pilot studies. Psychology of Addictive Behaviors, 27(2), 501–509. https://doi.org/10.1037/a0028215.
Hartwell, K. J., Hanlon, C. A., Li, X., Borckardt, J. J., Canterberry, M., Prisciandaro, J. J., et al. (2016). Individualized real-time fMRI neurofeedback to attenuate craving in nicotine-dependent smokers. Journal of Psychiatry and Neuroscience, 41(1), 48–55. https://doi.org/10.1503/jpn.140200.
Herremans, S. C., Vanderhasselt, M.-A., De Raedt, R., & Baeken, C. (2013). Reduced intra-individual reaction time variability during a go–NoGo task in detoxified alcohol-dependent patients after one right-sided dorsolateral prefrontal HF-rTMS session. Alcohol and Alcoholism, 48(5), 552–557. https://doi.org/10.1093/alcalc/agt054.
Hill, A. T., Rogasch, N. C., Fitzgerald, P. B., & Hoy, K. E. (2017). Effects of prefrontal bipolar and high-definition transcranial direct current stimulation on cortical reactivity and working memory in healthy adults. Neuroimage, 152, 142–157. https://doi.org/10.1016/j.neuroimage.2017.03.001.
Hone-Blanchet, A., Ciraulo, D. A., Pascual-Leone, A., & Fecteau, S. (2015). Noninvasive brain stimulation to suppress craving in substance use disorders: Review of human evidence and methodological considerations for future work. Neuroscience and Biobehavioral Reviews, 59, 184–200. https://doi.org/10.1016/j.neubiorev.2015.10.001.
Höppner, J., Broese, T., Wendler, L., Berger, C., & Thome, J. (2011). Repetitive transcranial magnetic stimulation (rTMS) for treatment of alcohol dependence. The World Journal of Biological Psychiatry, 12(sup1), 57–62.
Jamil, A., Batsikadze, G., Kuo, H. I., Labruna, L., Hasan, A., Paulus, W., & Nitsche, M. A. (2017). Systematic evaluation of the impact of stimulation intensity on neuroplastic after-effects induced by transcranial direct current stimulation. The Journal of Physiology, 595(4), 1273–1288. https://doi.org/10.1113/JP272738.
Jansen, J. M., Daams, J. G., Koeter, M. W. J., Veltman, D. J., van den Brink, W., & Goudriaan, A. E. (2013). Effects of non-invasive neurostimulation on craving: A meta-analysis. Neuroscience and Biobehavioral Reviews, 37(10 Pt 2), 2472–2480. https://doi.org/10.1016/j.neubiorev.2013.07.009.
Jones, F. W., & Holmes, D. S. (1976). Alcoholism, alpha production, and biofeedback. Journal of Consulting and Clinical Psychology, 44(2), 224–228.
Karch, S., Keeser, D., Hümmer, S., Paolini, M., Kirsch, V., Karali, T., et al. (2015). Modulation of craving related brain responses using real-time fMRI in patients with alcohol use disorder. PLoS One, 10(7), e0133034. https://doi.org/10.1371/journal.pone.0133034.
Keith, J. R., Rapgay, L., Theodore, D., Schwartz, J. M., & Ross, J. L. (2015). An assessment of an automated EEG biofeedback system for attention deficits in a substance use disorders residential treatment setting. Psychology of Addictive Behaviors : Journal of the Society of Psychologists in Addictive Behaviors, 29(1), 17–25. https://doi.org/10.1037/adb0000016.
Kim, D.-Y., Yoo, S.-S., Tegethoff, M., Meinlschmidt, G., & Lee, J.-H. (2015). The inclusion of functional connectivity information into fMRI-based neurofeedback improves its efficacy in the reduction of cigarette cravings. Journal of Cognitive Neuroscience, 27(8), 1552–1572. https://doi.org/10.1162/jocn_a_00802.
Kirsch, M., Gruber, I., Ruf, M., Kiefer, F., & Kirsch, P. (2016). Real-time functional magnetic resonance imaging neurofeedback can reduce striatal cue-reactivity to alcohol stimuli. Addiction Biology, 21(4), 982–992. https://doi.org/10.1111/adb.12278.
Kisely, S., Li, A., Warren, N., & Siskind, D. (2018). A systematic review and meta-analysis of deep brain stimulation for depression. Depression and Anxiety, 35(5), 468–480. https://doi.org/10.1002/da.22746.
Klauss, J., Penido Pinheiro, L. C., Silva Merlo, B. L., de Correia Santos, G. A., Fregni, F., Nitsche, M. A., & Miyuki Nakamura-Palacios, E. (2014). A randomized controlled trial of targeted prefrontal cortex modulation with tDCS in patients with alcohol dependence. International Journal of Neuropsychopharmacology, 17(11), 1793–1803.
Koob, G. F. (2015). The dark side of emotion: The addiction perspective. European Journal of Pharmacology, 753, 73–87. https://doi.org/10.1016/j.ejphar.2014.11.044.
Koob, G. F., & Volkow, N. D. (2009). Neurocircuitry of addiction. Neuropsychopharmacology, 35(1), 217–238. https://doi.org/10.1038/npp.2009.110.
Kuhn, J., Lenartz, D., Huff, W., Lee, S., Koulousakis, A., Klosterkoetter, J., & Sturm, V. (2007). Remission of alcohol dependency following deep brain stimulation of the nucleus accumbens: Valuable therapeutic implications? Journal of Neurology, Neurosurgery, and Psychiatry, 78(10), 1152–1153. https://doi.org/10.1136/jnnp.2006.113092.
Kuhn, J., Bauer, R., Pohl, S., Lenartz, D., Huff, W., Kim, E. H., et al. (2009). Observations on unaided smoking cessation after deep brain stimulation of the nucleus accumbens. European Addiction Research, 15(4), 196–201. https://doi.org/10.1159/000228930.
Kuhn, J., Gründler, T. O. J., Bauer, R., Huff, W., Fischer, A. G., Lenartz, D., et al. (2011). Successful deep brain stimulation of the nucleus accumbens in severe alcohol dependence is associated with changed performance monitoring. Addiction Biology, 16(4), 620–623. https://doi.org/10.1111/j.1369-1600.2011.00337.x.
Kuhn, J., Möller, M., Treppmann, J. F., Bartsch, C., Lenartz, D., Gruendler, T. O. J., et al. (2014). Deep brain stimulation of the nucleus accumbens and its usefulness in severe opioid addiction. Molecular Psychiatry, 19(2), 145–146. https://doi.org/10.1038/mp.2012.196.
Lackner, N., Unterrainer, H. F., Skliris, D., Wood, G., Wallner-Liebmann, S. J., Neuper, C., & Gruzelier, J. H. (2016). The effectiveness of visual short-time neurofeedback on brain activity and clinical characteristics in alcohol use disorders. Clinical EEG and Neuroscience, 47(3), 188–195. https://doi.org/10.1177/1550059415605686.
Li, X., Hartwell, K. J., Borckardt, J., Prisciandaro, J. J., Saladin, M. E., Morgan, P. S., et al. (2013). Volitional reduction of anterior cingulate cortex activity produces decreased cue craving in smoking cessation: A preliminary real-time fMRI study. Addiction Biology, 18(4), 739–748. https://doi.org/10.1111/j.1369-1600.2012.00449.x.
Ljubisavljevic, M., Maxood, K., Bjekic, J., Oommen, J., & Nagelkerke, N. (2016). Long-term effects of repeated prefrontal cortex transcranial direct current stimulation (tDCS) on food craving in normal and overweight young adults. Brain Stimulation, 9(6), 826–833.
Luigjes, J., Mantione, M., van den Brink, W., Schuurman, P. R., van den Munckhof, P., & Denys, D. (2011). Deep brain stimulation increases impulsivity in two patients with obsessive-compulsive disorder. International Clinical Psychopharmacology, 26(6), 338–340. https://doi.org/10.1097/YIC.0b013e32834af505.
Luigjes, J., van den Brink, W., Feenstra, M., van den Munckhof, P., Schuurman, P. R., Schippers, R., et al. (2012). Deep brain stimulation in addiction: A review of potential brain targets. Molecular Psychiatry, 17(6), 572–583. https://doi.org/10.1038/mp.2011.114.
Luigjes, J., de Kwaasteniet, B. P., de Koning, P. P., Oudijn, M. S., van den Munckhof, P., Schuurman, P. R., & Denys, D. (2013). Surgery for psychiatric disorders. World Neurosurgery, 80(3–4), S31.e17–S31.e28. https://doi.org/10.1016/j.wneu.2012.03.009.
Luigjes, J., van den Brink, W., Schuurman, P. R., Kuhn, J., & Denys, D. (2015). Is deep brain stimulation a treatment option for addiction? Addiction, 110(4), 547–548. https://doi.org/10.1111/add.12773.
Lujan, J. L., Chaturvedi, A., Malone, D. A., Rezai, A. R., Machado, A. G., & McIntyre, C. C. (2012). Axonal pathways linked to therapeutic and nontherapeutic outcomes during psychiatric deep brain stimulation. Human Brain Mapping, 33(4), 958–968. https://doi.org/10.1002/hbm.21262.
Lupi, M., Martinotti, G., Santacroce, R., Cinosi, E., Carlucci, M., Marini, S., Acciavatti, T., & di Giannantonio, M. (2017). Transcranial direct current stimulation in substance use disorders. The Journal of ECT, 33(3), 203–209. https://doi.org/10.1097/YCT.0000000000000401.
Maeda, F., Keenan, J. P., Tormos, J. M., Topka, H., & Pascual-Leone, A. (2000). Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Experimental Brain Research, 133(4), 425–430.
Malik, S., Jacobs, M., Cho, S. S., Boileau, I., Blumberger, D., Heilig, M., et al. (2017, November 23). Deep TMS of the insula using the H-coil modulates dopamine release: A crossover [11C] PHNO-PET pilot trial in healthy humans. Brain Imaging and Behavior, 1–12. https://doi.org/10.1007/s11682-017-9800-1.
Mantione, M., Nieman, D. H., Figee, M., & Denys, D. (2014). Cognitive-behavioural therapy augments the effects of deep brain stimulation in obsessive-compulsive disorder. Psychological Medicine, 44(16), 3515–3522. https://doi.org/10.1017/S0033291714000956.
Martínez-Rivera, F. J., Rodriguez-Romaguera, J., Lloret-Torres, M. E., Do Monte, F. H., Quirk, G. J., & Barreto-Estrada, J. L. (2016). Bidirectional modulation of extinction of drug seeking by deep brain stimulation of the ventral striatum. Biological Psychiatry, 80(9), 682–690. https://doi.org/10.1016/j.biopsych.2016.05.015.
Mclellan, A. T., Lewis, D. C., Brien, C. P. O., & Kleber, H. D. (2000). Drug dependence, a chronic medical illness: Implications for treatment, insurance, and Outcomes Evaluation. JAMA, 284(13), 1689–1695.
Merola, A., Romagnolo, A., Rizzi, L., Rizzone, M. G., Zibetti, M., Lanotte, M., et al. (2017). Impulse control behaviors and subthalamic deep brain stimulation in Parkinson disease. Journal of Neurology, 264(1), 40–48. https://doi.org/10.1007/s00415-016-8314-x.
Monte-Silva, K., Kuo, M. F., Hessenthaler, S., Fresnoza, S., Liebetanz, D., Paulus, W., & Nitsche, M. A. (2013). Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimulation, 6(3), 424–432. https://doi.org/10.1016/j.brs.2012.04.011.
Müller, U. J., Sturm V., Voges, J., Heinze, H. J., Galazky, I., Heldmann M et al. (2009). Successful treatment of chronic resistant alcoholism by deep brain stimulation of nucleus accumbens: first experience with three cases. Pharmacopsychiatry, 42, 288–291.
Müller, U. J., Sturm, V., Voges, J., Heinze, H. J., Galazky, I., Büntjen, L., et al. (2016). Nucleus Accumbens deep brain stimulation for alcohol addiction - safety and clinical long-term results of a pilot trial. Pharmacopsychiatry, 49(4), 170–173. https://doi.org/10.1055/s-0042-104507.
Nakamura-Palacios, E. M., Lopes, I. B., Souza, R. A., Klauss, J., Batista, E. K., Conti, C. L., et al. (2016). Ventral medial prefrontal cortex (vmPFC) as a target of the dorsolateral prefrontal modulation by transcranial direct current stimulation (tDCS) in drug addiction. Journal of Neural Transmission (Vienna), 123(10), 1179–1194. https://doi.org/10.1007/s00702-016-1559-9.
Naqvi, N. H., & Bechara, A. (2010). The insula and drug addiction: An interoceptive view of pleasure, urges, and decision-making. Brain Structure & Function, 214(5–6), 435–450. https://doi.org/10.1007/s00429-010-0268-7.
Naqvi, N. H., Rudrauf, D., Damasio, H., & Bechara, A. (2007). Damage to the insula disrupts addiction to cigarette smoking. Science, 315(5811), 531–534. https://doi.org/10.1126/science.1135926.
Nitsche, M. A., & Paulus, W. (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. Journal of Physiology, 527 Pt 3, 633–639.
Passini, F. T., Watson, C. G., Dehnel, L., Herder, J., & Watkins, B. (1977). Alpha wave biofeedback training therapy in alcoholics. Journal of Clinical Psychology, 33(1), 292–299.
Peniston, E. G., & Kulkosky, P. J. (1989). α-θ brainwave training and β-endorphin levels in alcoholics. Alcoholism: Clinical and Experimental Research, 13(2), 271–279. https://doi.org/10.1111/j.1530-0277.1989.tb00325.x.
Perera, T., George, M. S., Grammer, G., Janicak, P. G., Pascual-Leone, A., & Wirecki, T. S. (2016). The clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimulation, 9(3), 336–346. https://doi.org/10.1016/j.brs.2016.03.010.
Peters, J., Kalivas, P. W., & Quirk, G. J. (2009). Extinction circuits for fear and addiction overlap in prefrontal cortex. Learning & Memory, 16(5), 279–288. https://doi.org/10.1101/lm.1041309.
Pizzagalli, D. A., Holmes, A. J., Dillon, D. G., Goetz, E. L., Birk, J. L., Bogdan, R., et al. (2009). Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. The American Journal of Psychiatry, 166(6), 702–710. https://doi.org/10.1176/appi.ajp.2008.08081201.
Priori, A., Hallett, M., Rothwell, J. C., (2009). Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? Brain Stimulation 2(4),241-245
Rabey, J. M., Dobronevsky, E., Aichenbaum, S., Gonen, O., Marton, R. G., & Khaigrekht, M. (2013). Repetitive transcranial magnetic stimulation combined with cognitive training is a safe and effective modality for the treatment of Alzheimer’s disease: A randomized, double-blind study. Journal of Neural Transmission, 120(5), 813–819. https://doi.org/10.1007/s00702-012-0902-z.
Rawiji, V., Ciocca, M., Zacharia, A., Soares, D., Truong, D., Bikson, M., et al. (2017, March). TDCS changes in motor excitability are specific to orientation of current flow. Brain Stimulation, 11(2), 289–298. https://doi.org/10.1016/j.brs.2017.11.001.
Reuter, J., Raedler, T., Rose, M., Hand, I., Gläscher, J., & Büchel, C. (2005). Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nature Neuroscience, 8(2), 147–148. https://doi.org/10.1038/nn1378.
Riva-Posse, P., Choi, K. S., Holtzheimer, P. E., Crowell, A. L., Garlow, S. J., Rajendra, J. K., et al. (2018). A connectomic approach for subcallosal cingulate deep brain stimulation surgery: Prospective targeting in treatment-resistant depression. Molecular Psychiatry, 23(4), 843–849. https://doi.org/10.1038/mp.2017.59.
Root, D. H., Mejias-Aponte, C. A., Qi, J., & Morales, M. (2014). Role of glutamatergic projections from ventral tegmental area to lateral habenula in aversive conditioning. The Journal of Neuroscience, 34(42), 13906–13910. https://doi.org/10.1523/JNEUROSCI.2029-14.2014.
Rostami, R., & Dehghani-Arani, F. (2015). Neurofeedback training as a new method in treatment of crystal methamphetamine dependent patients: A preliminary study. Applied Psychophysiology and Biofeedback, 40(3), 151–161. https://doi.org/10.1007/s10484-015-9281-1.
Rouaud, T., Lardeux, S., Panayotis, N., Paleressompoulle, D., Cador, M., & Baunez, C. (2010). Reducing the desire for cocaine with subthalamic nucleus deep brain stimulation. Proceedings of the National Academy of Sciences of the United States of America, 107(3), 1196–1200. https://doi.org/10.1073/pnas.0908189107.
Russo, R., Twyman, P., Cooper, N. R., Fitzgerald, P. B., & Wallace, D. (2017). When you can, scale up: Large-scale study shows no effect of tDCS in an ambiguous risk-taking task. Neuropsychologia, 104, 133–143. https://doi.org/10.1016/j.neuropsychologia.2017.08.008.
Schippers, M. C., Bruinsma, B., Gaastra, M., Mesman, T. I., Denys, D., De Vries, T. J., & Pattij, T. (2017). Deep brain stimulation of the nucleus Accumbens Core affects trait impulsivity in a baseline-dependent manner. Frontiers in Behavioral Neuroscience, 11(March), 1–14. https://doi.org/10.3389/fnbeh.2017.00052.
Schmidt, J., & Martin, A. (2016). Neurofeedback against binge eating: A randomized controlled trial in a female subclinical threshold sample. European Eating Disorders Review, 24(5), 406–416. https://doi.org/10.1002/erv.2453.
Scott, W. C., Kaiser, D., Othmer, S., & Sideroff, S. I. (2005). Effects of an EEG biofeedback protocol on a mixed substance abusing population. The American Journal of Drug and Alcohol Abuse, 31(3), 455–469.
Segrave, R. A., Arnold, S., Hoy, K., & Fitzgerald, P. B. (2014). Concurrent cognitive control training augments the antidepressant efficacy of tDCS: A pilot study. Brain Stimulation, 7(2), 325–331. https://doi.org/10.1016/j.brs.2013.12.008.
Sesia, T., Temel, Y., Lim, L. W., Blokland, A., Steinbusch, H. W. M., & Visser-Vandewalle, V. (2008). Deep brain stimulation of the nucleus accumbens core and shell: Opposite effects on impulsive action. Experimental Neurology, 214(1), 135–139. https://doi.org/10.1016/j.expneurol.2008.07.015.
Sesia, T., Bulthuis, V., Tan, S., Lim, L. W., Vlamings, R., Blokland, A., et al. (2010). Deep brain stimulation of the nucleus accumbens shell increases impulsive behavior and tissue levels of dopamine and serotonin. Experimental Neurology, 225(2), 302–309. https://doi.org/10.1016/j.expneurol.2010.06.022.
Sheffer, C. E., Mennemeier, M., Landes, R. D., Bickel, W. K., Brackman, S., Dornhoffer, J., et al. (2013). Neuromodulation of delay discounting, the reflection effect, and cigarette consumption. Journal of Substance Abuse Treatment, 45(2), 206–214. https://doi.org/10.1016/j.jsat.2013.01.012.
Siebner, H. R., Peller, M., Willoch, F., Minoshima, S., Boecker, H., Auer, C., et al. (2000). Lasting cortical activation after repetitive TMS of the motor cortex: A glucose metabolic study. Neurology, 54(4), 956–963.
Smolders, R., Mazaheri, A., van Wingen, G., Figee, M., de Koning, P. P., & Denys, D. (2013). Deep brain stimulation targeted at the nucleus Accumbens decreases the potential for pathologic network communication. Biological Psychiatry, 74(10), e27–e28. https://doi.org/10.1016/j.biopsych.2013.03.012.
Sokhadze, T. M., Cannon, R. L., & Trudeau, D. L. (2008). EEG biofeedback as a treatment for substance use disorders: Review, rating of efficacy, and recommendations for further research. Applied Psychophysiology and Biofeedback, 33(1), 1–28. https://doi.org/10.1007/s10484-007-9047-5.
Spagnolo, P. A., & Goldman, D. (2017). Neuromodulation interventions for addictive disorders: Challenges, promise, and roadmap for future research. Brain, 140(5), aww284. https://doi.org/10.1093/brain/aww284.
Stagg, C. J., & Nitsche, M. A. (2011). Physiological basis of transcranial direct current stimulation. Neuroscientist, 17(1), 37–53. https://doi.org/10.1177/1073858410386614.
Stelten, B.M., Noblesse, L.H., Ackermans, L., Temel, Y., & Visser-Vandewalle, V. (2008). The neurosurgical treatment of addiction. Neurosurgical Focus, 25, E5. https://doi.org/10.3171/FOC/2008/25/7/E5.
Strube, W., Bunse, T., Nitsche, M. A., Nikolaeva, A., Palm, U., Padberg, F., et al. (2016). Bidirectional variability in motor cortex excitability modulation following 1 mA transcranial direct current stimulation in healthy participants. Physiological Reports, 4(15), e12884. https://doi.org/10.14814/phy2.12884.
Su, H., Zhong, N., Gan, H., Wang, J., Han, H., Chen, T., et al. (2017). High frequency repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex for methamphetamine use disorders: A randomised clinical trial. Drug and Alcohol Dependence, 175, 84–91.
Trojak, B., Meille, V., Achab, S., Lalanne, L., Poquet, H., Ponavoy, E., et al. (2015). Transcranial magnetic stimulation combined with nicotine replacement therapy for smoking cessation: A randomized controlled trial. Brain Stimulation, 8(6), 1168–1174.
Valencia-Alfonso, C.-E., Luigjes, J., Smolders, R., Cohen, M. X., Levar, N., Mazaheri, A., et al. (2012). Effective deep brain stimulation in heroin addiction: A case report with complementary intracranial electroencephalogram. Biological Psychiatry, 71(8), e35–e37. https://doi.org/10.1016/j.biopsych.2011.12.013.
van Hell, H. H., Vink, M., Ossewaarde, L., Jager, G., Kahn, R. S., & Ramsey, N. F. (2010). Chronic effects of cannabis use on the human reward system: An fMRI study. European Neuropsychopharmacology, 20(3), 153–163. https://doi.org/10.1016/j.euroneuro.2009.11.010.
Venniro, M., Caprioli, D., Zhang, M., Whitaker, L. R., Zhang, S., Warren, B. L., et al. (2017). The anterior insular cortex→central amygdala glutamatergic pathway is critical to relapse after contingency management. Neuron, 96(2), 414–427.e8. https://doi.org/10.1016/j.neuron.2017.09.024.
Verbruggen, F., Aron, A. R., Stevens, M. A., & Chambers, C. D. (2010). Theta burst stimulation dissociates attention and action updating in human inferior frontal cortex. Proceedings of the National Academy of Sciences of the United States of America, 107(31), 13966–13971. https://doi.org/10.1073/pnas.1001957107.
Voges, J., Müller, U., Bogerts, B., Münte, T., Heinze, H-J.(2013). Deep Brain Stimulation Surgery for Alcohol Addiction. World Neurosurgery 80(3-4).
Volkow, N. D., Wang, G.-J., Fowler, J. S., & Tomasi, D. (2012). Addiction circuitry in the human brain. Annual Review of Pharmacology and Toxicology, 52(1), 321–336. https://doi.org/10.1146/annurev-pharmtox-010611-134625.
Volkow, N. D., Wang, G. J., Tomasi, D., & Baler, R. D. (2013). Unbalanced neuronal circuits in addiction. Current Opinion in Neurobiology, 23(4), 639–648. https://doi.org/10.1016/j.conb.2013.01.002.
Vollstädt-Klein, S., Wichert, S., Rabinstein, J., Bühler, M., Klein, O., Ende, G., et al. (2010). Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction, 105(10), 1741–1749. https://doi.org/10.1111/j.1360-0443.2010.03022.x.
Walpoth, M., Hoertnagl, C., Mangweth-Matzek, B., Kemmler, G., Hinterhölzl, J., Conca, A., & Hausmann, A. (2008). Repetitive transcranial magnetic stimulation in bulimia nervosa: Preliminary results of a single-Centre, randomised, double-blind, sham-controlled trial in female outpatients. Psychotherapy and Psychosomatics, 77(1), 57–60.
Wang, J. X., & Voss, J. L. (2015). Long-lasting enhancements of memory and hippocampal-cortical functional connectivity following multiple-day targeted noninvasive stimulation. Hippocampus, 25(8), 877–883. https://doi.org/10.1002/hipo.22416.
Wiethoff, S., Hamada, M., & Rothwell, J. C. (2014). Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimulation, 7(3), 468–475. https://doi.org/10.1016/j.brs.2014.02.003.
Woods, A. J., Antal, A., Bikson, M., Boggio, P. S., Brunoni, A. R., Celnik, P., et al. (2016). A technical guide to tDCS, and related non-invasive brain stimulation tools. Clinical Neurophysiology, 127(2), 1031–1048. https://doi.org/10.1016/j.clinph.2015.11.012.
Wrase, J., Schlagenhauf, F., Kienast, T., Wüstenberg, T., Bermpohl, F., Kahnt, T., et al. (2007). Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. NeuroImage, 35(2), 787–794. https://doi.org/10.1016/j.neuroimage.2006.11.043.
Zack, M., Cho, S. S., Parlee, J., Jacobs, M., Li, C., Boileau, I., & Strafella, A. (2016). Effects of high frequency repeated transcranial magnetic stimulation and continuous Theta burst stimulation on gambling reinforcement, delay discounting, and Stroop interference in men with pathological gambling. Brain Stimulation, 9(6), 867–875. https://doi.org/10.1016/j.brs.2016.06.003.
Zhou, H., Xu, J., & Jiang, J. (2011). Deep brain stimulation of nucleus accumbens on heroin-seeking behaviors: A case report. Biological Psychiatry, 69(11), e41–e42. https://doi.org/10.1016/j.biopsych.2011.02.012.
Acknowledgements
We would like to thank Dr. Lucy Albertella for her work on the summary tables.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Martijn Figee and Damiaan Denys shared last authorship
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Luigjes, J., Segrave, R., de Joode, N. et al. Efficacy of Invasive and Non-Invasive Brain Modulation Interventions for Addiction. Neuropsychol Rev 29, 116–138 (2019). https://doi.org/10.1007/s11065-018-9393-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11065-018-9393-5