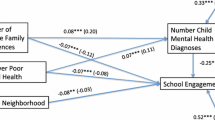
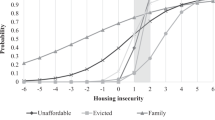
Abstract
Children raised in the profound deprivation associated with institutionalization are at elevated risk for negative outcomes across a host of social and cognitive domains. This risk appears to be mitigated by early foster care or adoption into a family setting. Although pervasive developmental problems have been noted in a substantial proportion of previously institutionalized children, marked variation exists in the nature and severity of these deficits. Increasing evidence suggests that institutional deprivation impacts the developing brain, potentially underlying the wide range of outcomes with which it is associated. In the current review we examine the neural consequences of institutionalization and genetic factors associated with differences in outcome in an effort to characterize the consequences of early deprivation at a neurobiological level. Although the effects of institutional deprivation have been studied for more than 50 years much remains unanswered regarding the pathways through which institutionalization impacts child development. Through a more complete and nuanced assessment of the neural correlates of exposure and recovery as well as a better understanding of the individual factors involved we will be better able to delineate the impact of early adversity in the setting of severe social deprivation.
Similar content being viewed by others
References
Albers, L. H., Johnson, D. E., Hostetter, M. K., Iverson, S., & Miller, L. C. (1997). Health of children adopted from the former Soviet Union and Eastern Europe. Comparison with preadoptive medical records. JAMA: The Journal of the American Medical Association, 278(11), 922–924.
American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders, 4th Edition (DSM-IV). Washington, DC: American Psychiatric.
Aragona, B., Liu, Y., et al. (2003). A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. The Journal of Neuroscience, 23(8), 3483–3490.
Babiloni, C., Frisoni, G., Steriade, M., Bresciani, L., Binetti, G., Del Percio, C., et al. (2006). Frontal white matter volume and delta EEG sources negatively correlate in awake subjects with mild cognitive impairment and Alzheimer’s disease. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 117(5), 1113–1129. doi:10.1016/j.clinph.2006.01.020.
Barr, C., Newman, T., et al. (2004). Interaction between serotonin transporter gene variation and rearing condition in alcohol preference and consumption in female primates. Archives of General Psychiatry, 61, 1146–1152.
Barry, R. J., Clarke, A. R., & Johnstone, S. J. (2003). A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clinical Neurophysiology, 114, 171–183.
Barry, R. J., Clarke, A. R., Johnstone, S. J., McCarthy, R., & Selikowitz, M. (2009). Electroencephalogram θ/β ratio and arousal in attention-deficit/hyperactivity disorder: Evidence of independent processes. Biological Psychiatry, 66, 398–40.
Bayley, N. (1993). Bayley scales of infant development. New York: Psychological Corp.
Beckett, C., Maughan, B., Rutter, M., Castle, J., Colvert, E., Groothues, C., et al. (2006). Do the effects of early severe deprivation on cognition persist into early adolescence? Findings from the English and Romanian adoptees study. Child Development, 77, 696–711.
Bell, M. A. (2002). Power changes in infant EEG frequency bands during a spatial working memory task. Psychophysiology, 39(4), 450–458. doi:10.1017.S0048577201393174.
Bell, M. A., & Wolfe, C. D. (2007). Changes in brain functioning from infancy to early childhood: evidence from EEG power and coherence working memory tasks. Developmental Neuropsychology, 31(1), 21–38. doi:10.1207/s15326942dn3101_2.
Bellgrove, M. A., Hawi, Z., Kirley, A., Gill, M., & Robertson, I. H. (2005). Dissecting the attention deficit hyperactivity disorder (ADHD) phenotype: sustained attention, response variability and spatial attentional asymmetries in relation to dopamine transporter (DAT1) genotype. Neuropsychologia, 43(13), 1847–1857. doi:10.1016/j.neuropsychologia.2005.03.011.
Bellgrove, M. A., & Mattingley, J. B. (2008). Molecular genetics of attention. Annals of the New York Academy of Sciences, 1129, 200–212. doi:10.1196/annals.1417.013.
Benoit, T. C., Jocelyn, L. J., Moddemann, D. M., & Embree, J. E. (1996). Romanian adoption. The Manitoba experience. Archives of Pediatrics & Adolescent Medicine, 150(12), 1278–1282.
Berton, O., McClung, C., et al. (2006). Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science, 311, 864–868.
Binder, E., Bradley, R., et al. (2008). Association of FKBP5 Polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA, 11, 1291–1305.
Boris, N. W., Hinshaw-Fuselier, S. S., et al. (2004). Comparing criteria for attachment disorders: establishing reliability and validity in high-risk samples. Journal of the American Academy of Child & Adolescent Psychiatry, 43(5), 568–577.
Bos, K. J., Zeanah, C. H., Smyke, A. T., Fox, N. A., & Nelson, C. A. (2010). Stereotypies in children with a history of early institutional care. Archives of Pediatrics & Adolescent Medicine, 164(5), 406–411. doi:10.1001/archpediatrics.2010.47
Burdick, K., Funke, B., et al. (2007). COMT genotype increases risk for bipolar I disorder and influences neurocognitive performance. Bipolar Disorder, 9, 370–376.
Caspi, A., Sugden, K., et al. (2003). Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science, 301, 386–389.
Caspi, A., Hariri, A., et al. (2010). Genetic sensitivity to the environment: the care of the serotonin transporter gene and its implications for studying complex diseases and traits. American Journal of Psychiatry Aia: 1–19.
Chanraud, S., Zahr, N. M., Sullivan, E. V., & Pfefferbaum, A. (2010). MR diffusion tensor imaging: a window into white matter integrity of the working brain. Neuropsychology Review, 20, 209–225.
Chisholm, K. (1998). A three year follow-up of attachment and indiscriminate friendliness in children adopted from Romanian orphanages. Child Development, 69(4), 1092–1096.
Chugani, H. T., Behen, M. E., Muzik, O., Juhász, C., Nagy, F., & Chugani, D. C. (2001). Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. NeuroImage, 14(6), 1290–1301. doi:10.1006/nimg.2001.0917.
Ciliax, B., Heilman, C., et al. (1995). The dopamine transporter: Immunochemical characterization and localization in brain. Journal of Neuroscience, 15, 1714–1723.
Clarke, A. R., Barry, R. J., McCarthy, R., & Selikowitz, M. (2001). Age and sex effects in the EEG: differences in two subtypes of attention-deficit/hyperactivity disorder. Clinical Neurophysiology, 112, 815–826.
Cyander, M. S., & Frost, B. J. (1999). Mechanisms of brain development: Neuronal sculpting by the physical and social environment. In D. P. Keating & C. Hertzman (Eds.), Developmental health and the wealth of nations: Social, biological, and educational dynamics. New York: Guilford.
de Haan, M., & Nelson, C. A. (1997). Recognition of the mother’s face by six-month-old infants: a neurobehavioral study. Child Development, 68(2), 187–210.
Dennis, W., & Najarian, P. (1957). Infant development under environmental handicap. Psychological Monographs, 71(7), 13.
Drury, S., Theall, K., et al. (2010). Modification of depression by the COMT val 158met polymorphisms in children exposed to early social deprivation. Child Abuse and Neglect.
Egan, M., Kojima, M., et al. (2003). The BDNF val66met polymorphisms affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell, 112, 257–269.
Egger, H. L., Ascher, B. H., & Angold, A. (1999). The preschool age psychiatric assessment: Version 1.1. Durham: Duke University Medical Center.
Egger, H., Erkanli, A., et al. (2006). Test-retest reliability of the Preschool Age Psychiatric Assessment (PAPA). The Journal of the American Academy of Child and Adolesc Psychiatry, 45, 538–549.
Eluvathingal, T. J., Chugani, H. T., Behen, M. E., Juhász, C., Muzik, O., Maqbool, M., et al. (2006). Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics, 117(6), 2093–2100. doi:10.1542/peds.2005-1727.
Fergusson, D. M., Horwood, L. J., & Lynskey, M. T. (1993). Prevalence and comorbidity of DSM-III-R diagnoses in a birth cohort of 15 year olds. Journal of the American Academy of Child & Adolescent Psychiatry, 32, 1127–1134.
Fox, S. E., Levitt, P., & Nelson, C. A. (2010). How the timing and quality of early experiences influence the development of brain architecture. Child Development, 81(1), 28–40. doi:10.1111/j.1467-8624.2009.01380.x.
Fox, N. F., Almas, A. N., Degnan, K. D., Nelson, C. A., & Zeanah, C. H. (2010). The effects of severe psychosocial deprivation on cognitive development at 8 years of age: Findings from the Bucharest Early Intervention Project. Manuscript submitted for publication
Funke, B., Malhotra, A., et al. (2005). COMT genetic variation confers risk for psychotic and affective disorders: a case control study. Behavior Brain Function, 1, 19.
Garris, P., & Wightman, R. (1994). Different kinetics govern dopaminergic transmission in the amygdala, prefrontal cortex, and striatum: an in vivo voltammetric study. Journal of Neuroscience, 14, 442–450.
Gauthier, I., & Nelson, C. A. (2001). The development of face expertise. Current Opinion in Neurobiology, 11(2), 219–224.
Giedd, J. N., Snell, J. W., Lange, N., Rajapakse, J. C., Casey, B. J., Kozuch, P. L., et al. (1996). Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cerebral Cortex (New York, N.Y.: 1991), 6(4), 551–560.
Gogos, J., Morgan, M., et al. (1998). Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proceedings of the National Academy of Science, 95, 9991–9996.
Grabe, H., Spitzer, C., et al. (2009). Serotonin transporter gene (SLC6A4) promoter polymorphism and the susceptibility to posttraumatic stress disorder in the general population. American Journal of Psychiatry, 166(8), 926–933.
Grady, D., Chi, H., et al. (2003). High prevalence of rare dopamine receptor D4 alleles in children diagnosed with attention-deficit hyperactivity disorder. Molecular Psychiatry, 8, 536–645.
Guillin, O., Diaz, J., et al. (2001). BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature, 411(6833), 86–89.
Guldberg, H., & Marsden, C. (1975). Catechol-O-methyl transferase: pharmacological aspects and physiological role. Pharmacological Reviews, 27, 135–206.
Gunnar, M. R., van Dulmen, M. H. M., & The International Adoption Project Team. (2007). Behavior problems in postinstitutionalized internationally adopted children. Development and Psychopathology, 19, 129–148.
Gunthert, K. C., Conner, T. S., et al. (2007). Serotonin transporter gene polymorphism (5-httlpr) and anxiety reactivity in daily life: a daily process approach to gene-environment interaction. Psychosomatic Medicine, 69, 762–768.
Heinz, A., Goldman, D., Jones, D. W., Palmour, R., Hommer, D., Gorey, J. G., Lee, K. S., et al. (2000). Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 22(2), 133–139. doi:10.1016/S0893-133X(99)00099-8
Hodges, J., & Tizard, B. (1989). Social and family relationships of ex-institutional adolescents. Journal of Child Psychology and Pschiatry, 30, 77–97.
Hoksbergen, R. A., ter Laak, J., van Dijkum, C., Rijk, S., Rijk, K., & Stoutjesdijk, F. (2003). Posttraumatic stress disorder in adopted children from Romania. American Journal of Orthopsychiatry, 73, 255–265.
Humphries, S. E., Talmud, P. J., et al. (2001). Apolipoprotein E4 and coronary heart disease in middle-aged men who smoke: a prospective study. The Lancet, 358(9276), 115–119.
Insel, T., & Fernald, R. (2004). How the brain processes social information: search for the social brain. Annual Review of Neuroscience, 27, 697–722.
Jacobsen, L. K., Staley, J. K., Zoghbi, S. S., Seibyl, J. P., Kosten, T. R., Innis, R. B., & Gelernter, J. (2000). Prediction of dopamine transporter binding availability by genotype: a preliminary report. The American Journal of Psychiatry, 157(10), 1700–1703.
Johnson, D. E., Guthrie, D., Smyke, A. T., Koga, S. F., Fox, N. A., Zeanah, C. H., et al. (2010). Growth and associations between auxology, caregiving environment, and cognition in socially deprived Romanian children randomized to foster vs ongoing institutional care. Archives of Pediatrics & Adolescent Medicine, 164(6), 507–516. doi:10.1001/archpediatrics.2010.56.
Karoum, F., Chrapusta, S., et al. (1994). 3-Methoxytryamine is the major metabolite of released dopamine in the rat frontal cortex: reassessment of the effects of antipsychotics on the dynamics of dopamine release and metabolism in the frontal cortex, nucleus accumbens, and striatum by a simple two pool model. Journal of Neurochemistry, 63, 972–979.
Kaufman, J., Yang, B., et al. (2004). Social supports and serotonin transporter gene moderate depression in maltreated children. PNAS, 101, 17316–11721.
Kaufman, J., Yang, B., et al. (2006). Brain-derived neurotrophic factor–5-HTTLPR gene interactions and environmental modifiers of depression in children. Biological Psychiatry, 59, 673–680.
Kendler, K., Kuhn, J., et al. (2005). The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Archives of General Psychiatry, 62, 529–535.
Kilpatrick, D., Koenen, K., et al. (2007). The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. American Journal of Psychiatry, 164, 1693–1699.
Kinsbourne, M. (1973). Minimal brain dysfunction as a neurodevelopmental lag. Annal of the New York Academy of Sciences, 205, 268–273.
Kreppner, J. M., O’Connor, T. G., & Rutter, M. (2001). Can inattention/overactivity be an institutional deprivation syndrome? Journal of Abnormal Child Psychology, 29(6), 513–528.
Kreppner, J. M., Rutter, M., Beckett, C., Castle, J., Colvert, E., Groothues, C., et al. (2007). Normality and impairment following profound early institutional deprivation: a longitudinal follow-up into early adolescence. Developmental Psychology, 43(4), 931–946. doi:10.1037/0012-1649.43.4.93.
Kumsta, R., Stevens, S., et al. (2010). 5HTT genotype moderates the influence of early institutional deprivation on emotional problems in adolescence: evidence from and English and Romanian Adoptee (ERA) study. The Journal of Child Psychology and Psychiatry.
Lachman, H., Papolos, D., et al. (1996). Human catechol-O-methyl transferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics, 6, 243–250.
Lavigne, J. V., LeBailly, S. A., Hopkins, J., Gouze, K. R., & Binns, H. J. (2009). The prevalence of ADHD, ODD, depression, and anxiety in a community sample of 4-year-olds. Journal of Clinical Child & Adolescent Psychology, 38(3), 315. doi:10.1080/15374410902851382.
Lesch, K., Bengel, D., et al. (1996). Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science, 274, 1527–1531.
Lotta, T., Vidgren, J., et al. (1995). Kinetics of a soluble and membrane-bound catechol-O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry, 34, 4204–4210.
Lyons-Ruth, K., Bureau, J., et al. (2009). Socially indiscriminate attachment behavior in the strange situation: Convergent and discriminant validity in relation to caregiving risk, later behavior problems, and attachment insecurity. Development and Psychopathology, 21, 355–372.
MacLean, K. (2003). The impact of institutionalization on child development. Development and Psychopathology, 15(4), 853–884.
Mann, C. A., Lubar, J. F., Zimmerman, A. W., Miller, C. A., & Muenchen, R. A. (1992). Quantitative analysis of EEG in boys with attention-deficit-hyperactivity disorder: Controlled study with clinical implications. Pediatric Neurology, 8, 30–36.
Marshall, P. J., & Fox, N. A. (2004). A comparison of the electroencephalogram between institutionalized and community children in Romania. Journal of Cognitive Neuroscience, 16(8), 1327–1338.
Marshall, P. J., Bar-Haim, Y., & Fox, N. A. (2002). Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology, 113(8), 1199–1208. doi:10.1016/S1388-2457(02)00163-3.
Marshall, P. J., Reeb, B. C., Fox, N. A., Nelson, C. A., & Zeanah, C. H. (2008). Effects of early intervention on EEG power and coherence in previously institutionalized children in Romania. Development and Psychopathology, 20(3), 861–880. doi:10.1017/S09545794080004.
Martinez, D., Broft, A., & Laruelle, M. (2001). Imaging neurochemical endophenotypes: promises and pitfalls. Pharmacogenomics, 2(3), 223–237. doi:10.1517/14622416.2.3.223.
Massat, I., Souery, D., et al. (2005). Association between COMT (Val158Met) functional polymorphism and early onset in patients with major depressive disorder in a European multicenter genetic association study. Molecular Psychiatry, 10(6), 598–605.
Matsuura, M., Okubo, Y., Toru, M., Kojima, T., He, Y., Hou, Y., et al. (1993). A cross-national EEG study of children with emotional and behavioural problems: a WHO collaborative study in the western pacific region. Biological Psychiatry, 34, 52–58.
McClay, J., Fanous, A., et al. (2006). Catechol-o-methyltransferase and the clinical features of psychosis. American Journal of Medical Genetics, 141B, 935–938.
McLaughlin, K. A., Fox, N. A., Zeanah, C. H., Sheridan, M. A., Marshall, P., & Nelson, C. A. (2010). Delayed maturation in brain electrical activity partially explains the association between early environmental deprivation and symptoms of attention-deficit/hyperactivity disorder. Biological Psychiatry, 68(4), 329–336. doi:10.1016/j.biopsych.2010.04.005.
Mehta, M. A., Golembo, N. I., Nosarti, C., Colvert, E., Mota, A., Williams, S. C. R., et al. (2009). Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 50(8), 943–951. doi:10.1111/j.1469-7610.2009.02084.
Michelhaugh, S. K., Fiskerstrand, C., Lovejoy, E., Bannon, M. J., & Quinn, J. P. (2001). The dopamine transporter gene (SLC6A3) variable number of tandem repeats domain enhances transcription in dopamine neurons. Journal of Neurochemistry, 79(5), 1033–1038.
Miller, L. C., & Hendrie, N. W. (2000). Health of children adopted from China. Pediatrics, 105(6), E76.
Miller, L. C., Kiernan, M. T., Mathers, M. I., & Klein-Gitelman, M. (1995). Developmental and nutritional status of internationally adopted children. Archives of Pediatrics & Adolescent Medicine, 149(1), 40–44.
Miller, L. C., Tseng, B., Tirella, L. G., Chan, W., & Feig, E. (2008). Health of children adopted from Ethiopia. Maternal and Child Health Journal, 12(5), 599–605. doi:10.1007/s10995-007-0274-4
Morishita, H., & Hensch, T. K. (2008). Critical period revisited: impact on vision. Current Opinion in Neurobiology, 18(1), 101–107. doi:10.1016/j.conb.2008.05.009.
Moulson, M. C., Fox, N. A., Zeanah, C. H., & Nelson, C. A. (2009). Early adverse experiences and the neurobiology of facial emotion processing. Developmental Psychology, 45(1), 17–30. doi:10.1037/a0014035.
Nelson, C. (2007). A neurobiological perspective on early human deprivation. Child Development Perspectives, 1(1), 13–18.
Nelson, C. A., Zeanah, C. H., Fox, N. A., Marshall, P. J., Smyke, A. T., & Guthrie, D. (2007). Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science (New York, N.Y.), 318(5858), 1937–1940. doi:10.1126/science.1143921.
O’Connor, T., & Rutter, M. (2000). Attachment disorder behavior following early severe deprivation: extension and longitudinal follow-up. Journal of the American Academy of Child & Adolescent Psychiatry, 39(6), 703.
O’Connor, T. G., Rutter, M., Beckett, C., Keaveney, L., & Kreppner, J. M. (2000). The effects of global severe privation on cognitive competence: extension and longitudinal follow-up. English and Romanian adoptees study team. Child Development, 71(2), 376–390.
O’hara, K., HNagai, M., et al. (1998). Low activity allele of catechol-o-methyltransferase gene and Japanese unipolar depression. Clinical Neuroscience, 9(7), 1305–1308.
Parker, S. W., & Nelson, C. A. (2005a). An event-related potential study of the impact of institutional rearing on face recognition. Development and Psychopathology, 17(3), 621–639. doi:10.1017/S0954579405050303.
Parker, S. W., & Nelson, C. A. (2005b). The impact of early institutional rearing on the ability to discriminate facial expressions of emotion: an event-related potential study. Child Development, 76(1), 54–72. doi:10.1111/j.1467-8624.2005.00829.x.
Pérez-Edgar, K., Bar-Haim, Y., et al. (2010). Variations in the serotonin-transporter gene are associated with attention bias patterns to positive and negative emotion faces. Biological Psychology, 83(3), 269–271.
Pezawas, L., Meyer-Lindenberg, A., et al. (2005). 5-httlpr polymorphism impacts human cingulate-amygdala interactions: a genetic suceptibility mechanism for depression. Nature Neuroscience, 8, 828–834.
Pollak, S. D., Nelson, C. A., Schlaak, M. F., Roeber, B. J., Wewerka, S. S., Wiik, K. L., et al. (2010). Neurodevelopmental effects of early deprivation in postinstitutionalized children. Child Development, 81(1), 224–236. doi:10.1111/j.1467-8624.2009.01391.x.
Rilling, J., Gutman, D., et al. (2002). A neural basis for social cooperation. 35, 395–405).
Risch, N., Herrell, R., et al. (2009). Interaction between the serotonin transporter gene (5-httlpr), stressful life events and risk of depression: a Meta-analysis. JAMA, 301(23), 2462–2471.
Rossini, P. M., Del Percio, C., Pasqualetti, P., Cassetta, E., Binetti, G., Dal Forno, G., et al. (2006). Conversion from mild cognitive impairment to Alzheimer’s disease is predicted by sources and coherence of brain electroencephalography rhythms. Neuroscience, 143(3), 793–803. doi:10.1016/j.neuroscience.2006.08.049.
Rotondo, A., Mazzanti, C., et al. (2002). Catechol-o-methyltransferase, serotonin transporter, and tryptophan hydroxylase gene polymrophisms in bipolar disorder patients with and without comorbid panic disorder. American Journal of Psychiatry, 159(1), 23–29.
Rutter, M. (2010). Gene-environment interplay. Depression and Anxiety, 27, 1–4.
Rutter, M., & English Romanian Adoptees (ERA) Study Team. (1998). Developmental catch-up, and deficit, following adoption after severe global early privation. Journal of Child Psychology and Psychiatry, 39, 465–476.
Rutter, M., & Sonuga-Barke, E. J. (2010). X. Conclusions: overview of findings from the era study, inferences, and research implications. Monographs of the Society for Research in Child Development, 75(1), 212–229. doi:10.1111/j.1540-5834.2010.00557.x.
Rutter, M., Andersen-Wood, L., Beckett, C., Bredenkamp, D., Castle, J., Groothues, C., Kreppner, J., et al. (1999). Quasi-autistic patterns following severe early global privation. English and Romanian Adoptees (ERA) Study Team. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 40(4), 537–549.
Rutter, M., Kreppner, J. M., O’Connor, T. G., & English Romanian Adoptees (ERA) Study Team. (2001). Specificity and heterogeneity in children’s responses to profound institutional privation. British Journal of Psychiatry, 17, 97–103.
Rutter, M., O’Connor, T. G., & English Romanian Adoptees (ERA) Study Team. (2004). Are there biological programming effects for psychological development? Findings from a study of Romanian adoptees. Developmental Psychology, 40, 81–94.
Rutter, M., Colvert, E., Kreppner, J., Beckett, C., Castle, J., Groothues, C., et al. (2007a). Early adolescent outcomes for institutionally-deprived and non-deprived adoptees. I: disinhibited attachment. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 48(1), 17–30. doi:10.1111/j.1469-7610.2006.01688.x.
Rutter, M., Beckett, C., et al. (2007b). Effects of profound early institutional deprivation: an overview of findings from a UK longitudinal study of Romanian adoptees. European Journal of Developmental Psychology, 4(3), 332–350.
Shaw, P., Eckstrand, K., Sharp, W., Blumenthal, J., Lerch, J. P., Greenstein, D., et al. (2007). Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proceedings of the National Academy of Sciences, 104, 19649–19654.
Smyke, A., Dumitrescu, A., et al. (2002). Attachment disturbances in young children I: the caretaking casualty continuum. Journal of the American Academy of Child & Adolescent Psychiatry, 41(8), 972–982.
Smyke, A. T., Zeanah, C. H., Fox, N. A., & Nelson, C. A. (2009). A new model of foster care for young children: the Bucharest early intervention project. Child and Adolescent Psychiatric Clinics of North America, 18(3), 721–734. doi:10.1016/j.chc.2009.03.003.
Stevens, S., Sonuga-Barke, E., et al. (2008). Inattention/overactivity following early severe institutional deprivation: presentation and associations in early adolescence. Journal of Abnormal Child Psychology, 36(3), 385–389.
Stevens, H., Leckman, J., et al. (2009). Risk and resilience: early manipulation of macaque social experience and persistent behavioral and neurophysiological outcomes. The Journal of the American Academy of Child and Adolescent Psychiatry, 48(2), 114–127.
Strathearn, L., Fonagy, P., et al. (2009). Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology, 34, 2655–2666.
Swain, J., Lorberbaum, J., et al. (2007). Brain basis of early parent-infant interactions: physiology, and in vivo functional neuroimaging studies. Journal of Child Psychology and Pschiatry, 48, 262–287.
Tottenham, N., & Sheridan, M. A. (2009). A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Frontiers in Human Neuroscience, 3, 68. doi:10.3389/neuro.09.068.2009.
Tottenham, N., Hare, T. A., Quinn, B. T., McCarry, T. W., Nurse, M., Gilhooly, T., et al. (2010). Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Science, 13(1), 46–61. doi:10.1111/j.1467-7687.2009.00852.x.
Uher, R., & McGuffin, P. (2008). The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Molecular Psychiatry, 13, 131–146.
Valdés-Hernández, P. A., Ojeda-González, A., Martínez-Montes, E., Lage-Castellanos, A., Virués-Alba, T., Valdés-Urrutia, L., et al. (2010). White matter architecture rather than cortical surface area correlates with the EEG alpha rhythm. NeuroImage, 49(3), 2328–2339. doi:10.1016/j.neuroimage.2009.10.030.
van den Dries, L., Juffer, F. J., van IJzendoorn, M. H., & Bakermans-Kranenburg, M. J. (2010). Infants’ physical and cognitive development after international adoption from foster care or institutions in China. Journal of Developmental & Behavioral Pediatrics, 31, 144–150.
Van Ijzendoorn, M., & Bakermans-Kraneburg, M. (2006). DRD4-7 repeat polymorphism moderates the association between maternal unresolved loss or trauma and infant disorganization. Attachment and Human Development, 8(4), 291–307.
Van Ijzendoorn, M. H., Bakermans-Kranenburg, M. J., & Juffer, F. (2007). Plasticity of growth in height, weight, and head circumference: meta-analytic evidence of massive catch-up after international adoption. Journal of Developmental and Behavioral Pediatrics: JDBP, 28(4), 334–343. doi:10.1097/DBP.0b013e31811320aa.
Vanderwert, R. E., Marshall, P. J., Nelson, C. A., Zeanah, C. H., & Fox, N. A. (2010). Timing of intervention affects brain electrical activity in children exposed to severe psychosocial neglect. PloS One, 5, e11415. doi:10.1371/journal.pone.0011415.
Wechsler, D. (2000). Wechsler preschool and primary scale of intelligence. San Antonio: Harcourt Assessment.
Wechsler, D. (2003). WISC-IV technical and interpretive manual. San Antonio: Psychological Corporation.
Willoughby, T., Curran, P. J., Costello, E. J., & Angold, A. (2000). Implications of early versus late onset of attention-deficit/hyperactivity disorder symptoms. Journal of the American Academy of Child & Adolescent Psychiatry, 39, 1512–1519.
Wolf, H., Jelic, V., Gertz, H., Nordberg, A., Julin, P., & Wahlund, L. (2003). A critical discussion of the role of neuroimaging in mild cognitive impairment. Acta Neurologica Scandinavica Supplementum, 179, 52–76.
Xie, P., Kranzler, H., et al. (2009). Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Archives of General Psychiatry, 66(11), 1201–1209.
Young, L., & Wang, Z. (2004). The neurobiology of pair bonding. Nature Neuroscience, 7(10), 1048–1054.
Zeanah, C., & Smyke, A. (2009). Disorders of attachment. Handbook of Infant Mental Health. C. Zeanah (pp. 421–434). New York: Guilford.
Zeanah, C. H., Nelson, C. A., Fox, N. A., Smyke, A. T., Marshall, P., Parker, S. W., et al. (2003). Designing research to study the effects of institutionalization on brain and behavioral development: the Bucharest Early Intervention Project. Development and Psychopathology, 15(4), 885–907.
Zeanah, C., Nelson, C., et al. (2003). Effects of institutionalization on brain and behavioral development: the Bucharest early intervention project. Development and Psychopathology, 15, 885–907.
Zeanah, C. H., Scheeringa, M., et al. (2004). Reactive attachment disorder in maltreated toddlers. Child Abuse & Neglect, 28(8), 877.
Zeanah, C., Smyke, A., et al. (2005). Attachment in institutionalized and community children in Romania. Child Development, 76(5), 1015–1028.
Zeanah, C., Egger, H., et al. (2009). Institutional rearing and psychiatric disorders in Romanian preschool children. American Journal of Psychiatry, 166, 777–785.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sheridan, M., Drury, S., McLaughlin, K. et al. Early Institutionalization: Neurobiological Consequences and Genetic Modifiers. Neuropsychol Rev 20, 414–429 (2010). https://doi.org/10.1007/s11065-010-9152-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11065-010-9152-8