Abstract
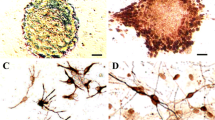
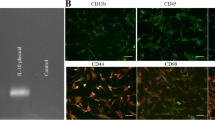
Transplantation of neural stem cells (NSCs) may be a potential strategy for traumatic brain injury treatment (TBI) due to their intrinsic advantages, such as cell replacement, secretion of neurotrophins and formation of functional synapses with host. However the underlying effects of transplanted NSCs on host micro-environment still need to be further elucidated. In this manuscript the effects of NSCs on release of neurotransmitter, survival of hippocampal neurons, reactivity of astrocytes and recovery of cognitive function after TBI were observed. The NSCs were isolated from cortex of neonatal Sprague–Dawley rat and then transplanted into injured brain regions caused by free-weight drop. The proliferation of astrocytes around injured sites were examined by GFAP immunofluorescent staining on 3, 7, 14 days after injury. The survival of neurons at CA1 regions of hippocampus toward contused regions was observed by HE staining on 3 and 14 days post-injury. The content of glutamic acid (Glu) and GABA in hippocampal tissues was examined on 1, 3, 7, 14, 28 days after injury by ELISA. On third day post-injury, hippocampal-dependent spatial memory was measured for 5 days without intermittent. NSCs in culture have the ability to proliferate and differentiate into different phenotypes of neural cells. After transplantation of NSCs, the proliferation of astrocytes around injured site was significantly inhibited compared to the injured group. At the same time the survival of neurons in hippocampal CA1 region were much more than those in injured group on 14 days post-injury. Meanwhile, the cognitive functions in NSC transplanted group was remarkably improved compared with injured group (p < 0.05). Furthermore, NSCs transplantation dramatically inhibited the release of Glu and maintained the content of GABA in injured hippocampal tissues on 1, 3, 7, 14, 28 days post-injury, which was of difference in statistics (p < 0.05). NSCs transplantation can effectively alleviate the formation of glial scar, enhance the survival of hippocampal neurons and improve cognitive function defects in rats with TBI. The underlying mechanism may be related to their effects on inhibiting the release of Glu and maintaining the content of GABA, so as to down-regulate excitotoxicity of neurotransmitter and improve the micro-environment in injured sites.






Similar content being viewed by others
References
David BA, Hal SW (2014) Emotional and behavioral dyscontrol after traumatic brain injury. Psychiatr Clin 37:31–53
Saeedeh S, Zohreh MP, Ghanbari A et al (2018) 9-cis-retinoic acid and 1,25-dihydroxy vitamin D3 improve the differentiation of neural stem cells into oligodendrocytes through the inhibition of the Notch and Wnt signaling pathways. Iran J Med Sci 43:523–532
You-Cui W, Qing-Jie X, Ying-Chun B et al (2014) Transplantation of olfactory ensheathing cells promotes the recovery of neurological functions in rats with traumatic brain injury associated with downregulation of Bad. Cytotherapy 16:1000–1010
Meghan OB, Pantelis T, Helen MB et al (2015) Neural progenitor cell transplantation promotes neuroprotection, enhances hippocampal neurogenesis, and improves cognitive outcomes after traumatic brain injury. Exp Neurol 264:67–81
Dong YC, Sin-Soo J (2018) Combination therapy of human bone marrow-derived mesenchymal stem cells and minocycline improves neuronal function in a rat middle cerebral artery occlusion model. Stem Cell Res Ther 9:309–310
Ma H, Yu B, Kong L et al (2011) Transplantation of neural stem cells enhances expression of synaptic protein and promotes functional recovery in a rat model of traumatic brain injury. Mol Med Rep. 4:849–856
Liu Yuan, Wang Li, Long Zai-yun (2013) Inhibiting PTEN protects hippocampal neurons against stretch injury by decreasing membrane translocation of AMPA receptor GluR2 subunit. PLoS ONE 8(e65431):1–8
Lei H, Jacqueline SC, Alena MY et al (2013) Tissue vulnerability is increased following repetitive mild traumatic brain injury in the rat. Brain Res 1499:109–120
Kaj B, John H, Henrik Z (2012) The neuropathology and neurobiology of traumatic brain injury. Neuron 76:888–899
Enci MK, Eng-Ang L, Jia L (2012) Microenvironment changes in mild traumatic brain injury. Brain Res Bull 87:359–372
Andrea SV, Tresa MRS, Alison C (2014) Cognitive changes and dementia risk after traumatic brain injury: implications for aging military personnel. Alzheimer’s Dementia 10:174–187
Frederick GS, Stefanie S, Kyle G et al (2014) Blood-based biomarkers for traumatic brain injury: evaluation of research approaches, available methods and potential utility from the clinician and clinical laboratory perspectives. Clin Biochem 47:876–888
Manojkumar V, Yonas AA, Stacey-Ann M et al (2013) Modulation of cholinergic pathways and inflammatory mediators in blast-induced traumatic brain injury. Chemico-Biol Interact 203:371–375
David T, Lital R, Vardit R et al (2013) Changes in mouse cognition and hippocampal gene expression observed in a mild physical- and blast-traumatic brain injury. Neurobiol Dis 54:1–11
Ashley MF, Karyn MF (2016) Hippocampal Wnt signaling: memory regulation and hormone interactions. Neuroscientist 22:278–294
Li W, Sen L, Yuan L et al (2017) Motor neuron degeneration following glycine-mediated excitotoxicity induces spastic paralysis after spinal cord ischemia/reperfusion injury in rabbit. Am J Transl Res 9:3411–3421
Margherita R, Giulietta R, Simona B et al (2014) Induced neural stem cells: methods of reprogramming and potential therapeutic applications. Prog Neurobiol 114:15–24
Jie G, He W, Yuan L (2014) Glutamate and GABA imbalance promotes neuronal apoptosis in hippocampus after stress. Med Sci Monit 20:499–512
Kaushal P, Dong S (2016) Strategies targeting endogenous neurogenic cell response to improve recovery following traumatic brain injury. Brain Res 1640:104–113
Tzu-Yun C, Ming-Hong C, Wen-Han C (2013) Neural stem cells encapsulated in a functionalized self-assembling peptide hydrogel for brain tissue engineering. Biomaterials 34:2005–2016
Xiang G, Jinhui C (2013) Moderate traumatic brain injury promotes neural precursor proliferation without increasing neurogenesis in the adult hippocampus. Exp Neurol 239:38–48
Wang L, Fu-xin W, Jing-sheng C et al (2014) Early administration of tumor necrosis factor-alpha antagonist promotes survival of transplanted neural stem cells and axon myelination after spinal cord injury in rats. Brain Res 1575:87–100
Simon MGB, Gregor-Alexander P, Raquel ACM et al (2015) Programming hippocampal neural stem/progenitor cells into oligodendrocytes enhances remyelination in the adult brain after injury. Cell Rep 11:1679–1685
Thompson Lachlan H, Björklun Anders (2015) Reconstruction of brain circuitry by neural transplants generated from pluripotent stem cells. Neurobiol Dis 79:28–40
Funding
Funding was provided by Qinnian Peiyu project of Military (Grant No. 16QNP105) and national natural scientific fund of China (Grant No. 81772066).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11064_2019_2897_MOESM5_ESM.ppt
Supplementary material 5 Supplemented Fig. 1. The labeled NSCs in brain tissue after transplantation. A. Location and migration of NSCs labeled by DAPI in the host brain 7 days post transplantation. (bar = 250 um). B. Location and migration of NSCs labeled by DAPI in the host brain 14 days post transplantation. (bar = 250 um) (PPT 973 kb)
Rights and permissions
About this article
Cite this article
Luo, Ml., Pan, L., Wang, L. et al. Transplantation of NSCs Promotes the Recovery of Cognitive Functions by Regulating Neurotransmitters in Rats with Traumatic Brain Injury. Neurochem Res 44, 2765–2775 (2019). https://doi.org/10.1007/s11064-019-02897-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02897-z