Abstract
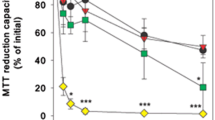
Copper oxide nanoparticles (CuO-NPs) dispersions are known for their high cell toxic potential but contaminating copper ions in such dispersions are a major hurdle in the investigation of specific nanoparticle-mediated toxicity. In order to distinguish between the adverse effects exhibited by CuO-NPs and/or by contaminating ionic copper, the membrane-impermeable copper chelator bathocuproine disulfonate (BCS) was added in a low molar ratio (20% of the total copper applied) in order to chelate the copper ions that had been released extracellularly from the CuO-NPs before or during the incubation. Physicochemical characterization of synthesized CuO-NPs revealed that the presence of this low concentration of BCS did not alter the size or zeta potential of the CuO-NPs. Application of CuO-NPs to C6 glioma cells and primary astrocytes induced a concentration- and temperature-dependent copper accumulation which was accompanied by a severe loss in cell viability. The adverse consequences of the CuO-NP application were not affected by the presence of 20% BCS, while the copper accumulation and cell toxicity observed after application of ionic copper were significantly lowered in the presence of BCS. These results demonstrate that for the experimental conditions applied the adverse consequences of an exposure of cultured glial cells to dispersions of CuO-NPs are mediated by accumulated NPs and not caused by the uptake of contaminating copper ions.








Similar content being viewed by others
References
Chauhan M, Sharma B, Kumar R, Chaudhary GR, Hassan AA, Kumar S (2019) Green synthesis of CuO nanomaterials and their proficient use for organic waste removal and antimicrobial application. Environ Res 168:85–95
Khatami M, Alijani HQ, Sharifi I (2018) Biosynthesis of bimetallic and core–shell nanoparticles: their biomedical applications–a review. IET Nanobiotechnol 12:879–887
Kadiyala U, Kotov NA, VanEpps JS (2018) Antibacterial metal oxide nanoparticles: challenges in interpreting the literature. Curr Pharm Des 24:896–903
Titma T, Shimmo R, Siigur J, Kahru A (2016) Toxicity of antimony, copper, cobalt, manganese, titanium and zinc oxide nanoparticles for the alveolar and intestinal epithelial barrier cells in vitro. Cytotechnology 68:2363–2377
Líbalová H, Costa PM, Olsson M, Farcal L, Ortelli S, Blosi M, Topinka J, Costa AL, Fadeel B (2018) Toxicity of surface-modified copper oxide nanoparticles in a mouse macrophage cell line: Interplay of particles, surface coating and particle dissolution. Chemosphere 196:482–493
Zhang J, Zou Z, Wang B, Xu G, Wu Q, Zhang Y, Yuan Z, Yang X, Yu C (2018) Lysosomal deposition of copper oxide nanoparticles triggers HUVEC cells death. Biomaterials 161:228–239
Bulcke F, Thiel K, Dringen R (2014) Uptake and toxicity of copper oxide nanoparticles in cultured primary brain astrocytes. Nanotoxicology 8:775–785
Kukia NR, Abbasi A, Froushani SMA (2018) Copper oxide nanoparticles stimulate cytotoxicity and apoptosis in glial cancer cell line. Dhaka Univ J Pharm Sci 17:105–111
Vinardell M, Mitjans M (2018) Metal/metal oxide nanoparticles for cancer therapy. In: Goncalves G, Tobias G (eds) Nanooncology. Springer, Cham, pp 341–364
Zhou Y, Peng Z, Seven ES, Leblanc RM (2018) Crossing the blood-brain barrier with nanoparticles. J Control Release 270:290–303
Khalid S, Afzal N, Khan JA, Hussain Z, Qureshi AS, Anwar H, Jamil Y (2018) Antioxidant resveratrol protects against copper oxide nanoparticle toxicity in vivo. Naunyn-Schmiedeberg's Arch Pharmacol 391:1053–1062
Li X, Sun W, An L (2018) Nano-CuO impairs spatial cognition associated with inhibiting hippocampal long-term potentiation via affecting glutamatergic neurotransmission in rats. Toxicol Ind Health 34:409–421
Lian D, Chonghua Z, Wen G, Hongwei Z, Xuetao B (2017) Label-free and dynamic monitoring of cytotoxicity to the blood–brain barrier cells treated with nanometre copper oxide. IET Nanobiotechnol 11:948–956
Prabhu BM, Ali SF, Murdock RC, Hussain SM, Srivatsan M (2010) Copper nanoparticles exert size and concentration dependent toxicity on somatosensory neurons of rat. Nanotoxicology 4:150–160
Mahmoud A, Elif GE, Gül O (2016) Copper (II) Oxide nanoparticles induce high toxicity in human neuronal cell. Glob J Med Res 16:7–14
Conway JR, Adeleye AS, Gardea-Torresdey J, Keller AA (2015) Aggregation, dissolution, and transformation of copper nanoparticles in natural waters. Environ Sci Technol 49:2749–2756
Midander K, Wallinder IO, Leygraf C (2007) In vitro studies of copper release from powder particles in synthetic biological media. Environ Pollut 145:51–59
Midander K, Cronholm P, Karlsson HL, Elihn K, Möller L, Leygraf C, Wallinder IO (2009) Surface characteristics, copper release, and toxicity of nano-and micrometer-sized copper and copper (II) oxide particles: a cross-disciplinary study. Small 5:389–399
Semisch A, Ohle J, Witt B, Hartwig A (2014) Cytotoxicity and genotoxicity of nano-and microparticulate copper oxide: role of solubility and intracellular bioavailability. Part Fibre Toxicol 11:10–26
Jeong J, Kim S-H, Lee S, Lee D-K, Han Y, Jeon S, Cho W-S (2018) Differential contribution of constituent metal ions to the cytotoxic effects of fast-dissolving metal-oxide nanoparticles. Front Pharmacol 9:15–25
Wang D, Lin Z, Wang T, Yao Z, Qin M, Zheng S, Lu W (2016) Where does the toxicity of metal oxide nanoparticles come from: the nanoparticles, the ions, or a combination of both? J Hazard Mater 308:328–334
Joshi A, Rastedt W, Faber K, Schultz AG, Bulcke F, Dringen R (2016) Uptake and toxicity of copper oxide nanoparticles in C6 glioma cells. Neurochem Res 41:3004–3019
Cho W-S, Duffin R, Poland CA, Duschl A, Oostingh GJ, MacNee W, Bradley M, Megson IL, Donaldson K (2012) Differential pro-inflammatory effects of metal oxide nanoparticles and their soluble ions in vitro and in vivo; zinc and copper nanoparticles, but not their ions, recruit eosinophils to the lungs. Nanotoxicology 6:22–35
Chen S-H, Lin J-K, Liu S-H, Liang Y-C, Lin-Shiau S-Y (2007) Apoptosis of cultured astrocytes induced by the copper and neocuproine complex through oxidative stress and JNK activation. Toxicol Sci 102:138–149
Kim BH, Yang J, Lee D, Choi BK, Hyeon T, Park J (2018) Liquid-phase transmission electron microscopy for studying colloidal inorganic nanoparticles. Adv Mater 30:1703316
Michen B, Geers C, Vanhecke D, Endes C, Rothen-Rutishauser B, Balog S, Petri-Fink A (2015) Avoiding drying-artifacts in transmission electron microscopy: Characterizing the size and colloidal state of nanoparticles. Sci Rep 5:9793
Benda P, Lightbody J, Sato G, Levine L, Sweet W (1968) Differentiated rat glial cell strain in tissue culture. Science 161:370–371
Stapelfeldt K, Ehrke E, Steinmeier J, Rastedt W, Dringen R (2017) Menadione-mediated WST1 reduction assay for the determination of metabolic activity of cultured neural cells. Anal Biochem 538:42–52
Tulpule K, Hohnholt MC, Hirrlinger J, Dringen R (2014) Primary cultures of astrocytes and neurons as model systems to study the metabolism and metabolite export from brain cells. In: Hirrlinger J, Waagepetersen H (eds) Neuromethods: brain energy metabolism. Springer, New York, pp 45–72
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Chen L, Xue X, Jiang D, Yang J, Zhao B, Han XX, Mee Jung Y (2016) A turn-on resonance Raman scattering (BCS/Cu+) sensor for quantitative determination of proteins. Appl Spectrosc 70:355–362
Bulcke F, Santofimia-Castaño P, Gonzalez-Mateos A, Dringen R (2015) Modulation of copper accumulation and copper-induced toxicity by antioxidants and copper chelators in cultured primary brain astrocytes. J Trace Elem Med Biol 32:168–176
Stark WJ (2011) Nanoparticles in biological systems. Angew Chem 50:1242–1258
Chen D, Darabedian N, Li Z, Kai T, Jiang D, Zhou F (2016) An improved Bathocuproine assay for accurate valence identification and quantification of copper bound by biomolecules. Anal Biochem 497:27–35
Arratia F, Olivares-Ferretti P, García-Rodríguez A, Marcos R, Carmona ER (2019) Comparative toxic effects of copper-based nanoparticles and their microparticles in Daphnia magna by using natural freshwater media. New Zeal J Mar Fresh 53:460–469
Henson TE, Navratilova J, Tennant AH, Bradham KD, Rogers KR, Hughes MF (2019) In vitro intestinal toxicity of copper oxide nanoparticles in rat and human cell models. Nanotoxicology 13:795–811
Jurašin DD, Ćurlin M, Capjak I, Crnković T, Lovrić M, Babič M, Horák D, Vrček IV, Gajović S (2016) Surface coating affects behavior of metallic nanoparticles in a biological environment. Beilstein J Nanotechnol 7:246–262
Ortelli S, Costa AL, Blosi M, Brunelli A, Badetti E, Bonetto A, Hristozov D, Marcomini A (2017) Colloidal characterization of CuO nanoparticles in biological and environmental media. Environ Sci Nano 4:1264–1272
Geppert M, Petters C, Thiel K, Dringen R (2013) The presence of serum alters the properties of iron oxide nanoparticles and lowers their accumulation by cultured brain astrocytes. J Nanopart Res 15:1349–1364
Scheiber IF, Mercer JF, Dringen R (2010) Copper accumulation by cultured astrocytes. Neurochem Int 56:451–460
Tiffany-Castiglioni E, Qian Y (2001) Astroglia as metal depots: molecular mechanisms for metal accumulation, storage and release. Neurotoxicology 22:577–592
Bulcke F, Dringen R (2016) Handling of copper and copper oxide nanoparticles by astrocytes. Neurochem Res 41:33–43
Behzadi S, Serpooshan V, Tao W, Hamaly MA, Alkawareek MY, Dreaden EC, Brown D, Alkilany AM, Farokhzad OC, Mahmoudi M (2017) Cellular uptake of nanoparticles: journey inside the cell. Chem Soc Rev 46:4218–4244
Willmann W, Dringen R (2018) Monitoring of the cytoskeleton-dependent intracellular trafficking of fluorescent iron oxide nanoparticles by nanoparticle pulse-chase experiments in C6 glioma cells. Neurochem Res 43:2055–2071
Geppert M, Hohnholt MC, Thiel K, Nürnberger S, Grunwald I, Rezwan K, Dringen R (2011) Uptake of dimercaptosuccinate-coated magnetic iron oxide nanoparticles by cultured brain astrocytes. Nanotechnology 22:145101–145111
Masuoka J, Saltman P (1994) Zinc (II) and copper (II) binding to serum albumin. A comparative study of dog, bovine, and human albumin. J Biol Chem 269:25557–25561
Peters T, Blumenstock FA (1967) Copper-binding properties of bovine serum albumin and its amino-terminal peptide fragment. J Biol Chem 242:1574–1578
Scheiber IF, Mercer JF, Dringen R (2014) Metabolism and functions of copper in brain. Prog Neurobiol 116:33–57
Akhtar MJ, Kumar S, Alhadlaq HA, Alrokayan SA, Abu-Salah KM, Ahamed M (2016) Dose-dependent genotoxicity of copper oxide nanoparticles stimulated by reactive oxygen species in human lung epithelial cells. Toxicol Ind Health 32:809–821
Angelé-Martínez C, Nguyen KVT, Ameer FS, Anker JN, Brumaghim JL (2017) Reactive oxygen species generation by copper (II) oxide nanoparticles determined by DNA damage assays and EPR spectroscopy. Nanotoxicology 11:278–288
Huang Y-W, Cambre M, Lee H-J (2017) The toxicity of nanoparticles depends on multiple molecular and physicochemical mechanisms. Int J Mol Sci 18:2702–2715
Chang Y-N, Zhang M, Xia L, Zhang J, Xing G (2012) The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials 5:2850–2871
Macomber L, Imlay JA (2009) The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci 106:8344–8349
Saporito-Magriñá CM, Musacco-Sebio RN, Andrieux G, Kook L, Orrego MT, Tuttolomondo MV, Desimone MF, Boerries M, Borner C, Repetto MG (2018) Copper-induced cell death and the protective role of glutathione: the implication of impaired protein folding rather than oxidative stress. Metallomics 10:1743–1754
Noventa S, Hacker C, Rowe D, Elgy C, Galloway T (2018) Dissolution and bandgap paradigms for predicting the toxicity of metal oxide nanoparticles in the marine environment: an in vivo study with oyster embryos. Nanotoxicology 12:63–78
Djurišić AB, Leung YH, Ng AM, Xu XY, Lee PK, Degger N, Wu R (2015) Toxicity of metal oxide nanoparticles: mechanisms, characterization, and avoiding experimental artefacts. Small 11:26–44
Acknowledgements
Arundhati Joshi would like to thank the Hans-Böckler Foundation for a PhD fellowship at the Graduate School NanoCompetence at the University of Bremen, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Joshi, A., Thiel, K., Jog, K. et al. Uptake of Intact Copper Oxide Nanoparticles Causes Acute Toxicity in Cultured Glial Cells. Neurochem Res 44, 2156–2169 (2019). https://doi.org/10.1007/s11064-019-02855-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02855-9