Abstract
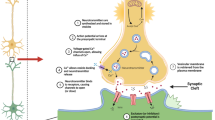
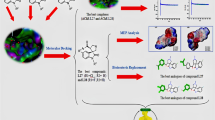
Inhibitors of acetylcholinesterase (AChE), which have an important role in the prevention of excessive AChE activity and β-amyloid (Aβ) formation are widely used in the symptomatic treatment of Alzheimer's disease (AD). The inhibitory effect of anesthetic agents on AChE was determined by several approaches, including binding mechanisms, molecular docking and kinetic analysis. Inhibitory effect of intravenous anesthetics on AChE as in vitro and in vivo have been discovered. The midazolam, propofol and thiopental have shown competitive inhibition type (midazolam > propofol > thiopental) and Ki values were found to be 3.96.0 ± 0.1, 5.75 ± 0.12 and 29.65 ± 2.04 µM, respectively. The thiopental and midazolam showed inhibition effect on AChE in vitro, whereas they showed activation effect in vivo when they are combined together. The order of binding of the drugs to the active site of the 4M0E receptor was found to be midazolam > propofol > thiopental. This study on anesthetic agents that are now widely used in surgical applications, have provided a molecular basis for investigating the drug-enzyme interactions mechanism. In addition, the study is important in understanding the molecular mechanism of inhibitors that are effective in the treatment of AD.




Similar content being viewed by others
References
Santamaria LB, Schifilliti D, La Torre D, Fodale V (2010) Drugs of anaesthesia and cancer. Surg Oncol. https://doi.org/10.1016/j.suronc.2009.03.007
Kvolik S, Glavas-Obrovac L, Bares V, Karner I (2005) Effects of inhalation anesthetics halothane, sevoflurane, and isoflurane on human cell lines. Life Sci. https://doi.org/10.1016/j.lfs.2004.12.052
Mammoto T, Mukai M, Mammoto A et al (2002) Intravenous anesthetic, propofol inhibits invasion of cancer cells. Cancer Lett. https://doi.org/10.1016/S0304-3835(02)00210-0
Inal M, Alatas O, Kural T, Sevin B (1994) Oxygen free radicals in erythrocytes during open heart operation. J Cardiovasc Surg 35:147–150
Weiss M, Buhl R, Birkhahn A et al (1994) Do barbiturates and their solutions suppress FMLP-induced neutrophil chemiluminescence? Eur J Anaesthesiol 11:371–379
Kelicen P, Ismailoglu UB, Erdemli O, Sahin-Erdemli I (1997) The effect of propofol and thiopentone on impairment by reactive oxygen species of endothelium-dependent relaxation in rat aortic rings. Eur J Anaesthesiol. https://doi.org/10.1097/00003643-199705000-00015
Vasileiou I, Xanthos T, Koudouna E et al (2009) Propofol: a review of its non-anaesthetic effects. Eur J Pharmacol. https://doi.org/10.1016/j.ejphar.2009.01.007
Lee Y-M, Song BC, Yeum K-J (2015) Impact of volatile anesthetics on oxidative stress and inflammation. Biomed Res Int. https://doi.org/10.1155/2015/242709
Calabrese V, Giordano J, Signorile A et al (2016) Major pathogenic mechanisms in vascular dementia: roles of cellular stress response and hormesis in neuroprotection. J Neurosci Res. https://doi.org/10.1002/jnr.23925
Işık M, Beydemir Ş, Yılmaz A et al (2017) Oxidative stress and mRNA expression of acetylcholinesterase in the leukocytes of ischemic patients. Biomed Pharmacother. https://doi.org/10.1016/j.biopha.2017.01.003
Fang J, Wu P, Yang R et al (2014) Inhibition of acetylcholinesterase by two genistein derivatives: kinetic analysis, molecular docking and molecular dynamics simulation. Acta Pharm Sin B. https://doi.org/10.1016/j.apsb.2014.10.002
Anand P, Singh B (2013) A review on cholinesterase inhibitors for Alzheimer’s disease. Arch Pharm Res. https://doi.org/10.1007/s12272-013-0036-3
Sabbagh MN (2009) Drug development for Alzheimer’s disease: where are we now and where are we headed? Am J Geriatr Pharmacother. https://doi.org/10.1016/j.amjopharm.2009.06.003
Citron M (2010) Alzheimer’s disease: strategies for disease modification. Nat Rev Drug Discov. https://doi.org/10.1038/nrd2896
Chaudière J, Ferrari-Iliou R (1999) Intracellular antioxidants: From chemical to biochemical mechanisms. Food Chem Toxicol. https://doi.org/10.1016/S0278-6915(99)00090-3
Göcer H, Akincioʇlu A, Göksu S et al (2015) Carbonic anhydrase and acetylcholinesterase inhibitory effects of carbamates and sulfamoylcarbamates. J Enzyme Inhib Med Chem. https://doi.org/10.3109/14756366.2014.928704
Alici HA, Ekinci D, Beydemir Ş (2008) Intravenous anesthetics inhibit human paraoxonase-1 (PON1) activity in vitro and in vivo. Clin Biochem. https://doi.org/10.1016/j.clinbiochem.2008.06.017
Ellman GL, Courtney DK, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Taslimi P, Kandemir FM, Demir Y et al (2019) The antidiabetic and anticholinergic effects of chrysin on cyclophosphamide-induced multiple organ toxicity in rats: pharmacological evaluation of some metabolic enzyme activities. J Biochem Mol Toxicol. https://doi.org/10.1002/jbt.22313
Wright DL, Plummer DT (2015) Multiple forms of acetylcholinesterase from human erythrocytes. Biochem J. https://doi.org/10.1042/bj1330521
Yamali C, Gul HI, Ece A et al (2018) Synthesis, molecular modeling, and biological evaluation of 4-[5-aryl-3-(thiophen-2-yl)-4,5-dihydro-1H-pyrazol-1-yl] benzenesulfonamides toward acetylcholinesterase, carbonic anhydrase I and II enzymes. Chem Biol Drug Des. https://doi.org/10.1111/cbdd.13149
Demir Y, Işık M, Gülçin İ, Beydemir Ş (2017) Phenolic compounds inhibit the aldose reductase enzyme from the sheep kidney. J Biochem Mol Toxicol. https://doi.org/10.1002/jbt.21935
Halgren TA, Murphy RB, Friesner RA et al (2004) Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J Med Chem. https://doi.org/10.1021/jm030644s
Türkeş C (2019) Investigation of potential paraoxonase-I inhibitors by kinetic and molecular docking studies: chemotherapeutic drugs. Protein Pept Lett. https://doi.org/10.2174/0929866526666190226162225
Madhavi Sastry G, Adzhigirey M, Day T et al (2013) Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J Comput Aided Mol Des. https://doi.org/10.1007/s10822-013-9644-8
Friesner RA, Banks JL, Murphy RB et al (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem. https://doi.org/10.1021/jm0306430
Harder E, Damm W, Maple J et al (2016) OPLS3: a force field providing broad coverage of drug-like small molecules and proteins. J Chem Theory Comput. https://doi.org/10.1021/acs.jctc.5b00864
Ansley DM, Lee JU, Godin DV et al (1998) Propofol enhances red cell antioxidant capacity in swine and humans. Can J Anaesth. https://doi.org/10.1007/BF03012908
Hans P, Deby-Dupont G, Deby C et al (1997) Increase in antioxidant capacity of plasma during propofol anesthesia. J Neurosurg Anesthesiol. https://doi.org/10.1097/00008506-199707000-00006
Manataki AD, Tselepis AD, Glantzounis GK et al (2001) Lipid peroxidation and the use of emulsified propofol in laparoscopic surgery. Surg Endosc. https://doi.org/10.1007/s004640090104
Stratford N, Murphy P (1998) Antioxidant activity of propofol in blood from anaesthetized patients. Eur J Anaesthesiol. https://doi.org/10.1097/00003643-199803000-00006
Yagmurdur H, Cakan T, Bayrak AK et al (2004) The effects of etomidate, thiopental, and propofol in induction on hypoperfusion-reperfusion phenomenon during laparoscopic cholecystectomy. Acta Anaesthesiol Scand. https://doi.org/10.1111/j.0001-5172.2004.00417.x
Harman F, Hasturk AE, Yaman M et al (2012) Neuroprotective effects of propofol, thiopental, etomidate, and midazolam in fetal rat brain in ischemia-reperfusion model. Child’s Nerv Syst. https://doi.org/10.1007/s00381-012-1782-0
Calabrese V, Giordano J, Crupi R et al (2017) Hormesis, cellular stress response and neuroinflammation in schizophrenia: early onset versus late onset state. J Neurosci Res. https://doi.org/10.1002/jnr.23967
Runzer TD, Ansley DM, Godin DV, Chambers GK (2002) Tissue antioxidant capacity during anesthesia: propofol enhances in vivo red cell and tissue antioxidant capacity in a rat model. Anesth Analg. https://doi.org/10.1213/00000539-200201000-00017
Calabrese EJ (2019) The linear No-Threshold (LNT) dose response model: a comprehensive assessment of its historical and scientific foundations. Chem Biol Interact. https://doi.org/10.1016/j.cbi.2018.11.020
Nishina K, Akamatsu H, Mikawa K et al (1998) The inhibitory effects of thiopental, midazolam, and ketamine on human neutrophil functions. Anesth Analg. https://doi.org/10.1097/00000539-199801000-00032
Xue Q-S, Yu B-W, Wang Z-J, Chen H-Z (2004) Effects of ketamine, midazolam, thiopental, and propofol on brain ischemia injury in rat cerebral cortical slices. Acta Pharmacol Sin 25:115–120
Sánchez-Conde P, Rodríguez-López JM, Nicolás JL et al (2008) The comparative abilities of propofol and sevoflurane to modulate inflammation and oxidative stress in the kidney after aortic cross-clamping. Anesth Analg. https://doi.org/10.1213/ane.0b013e318160580b
Chillemi R, Cardullo N, Greco V et al (2015) Synthesis of amphiphilic resveratrol lipoconjugates and evaluation of their anticancer activity towards neuroblastoma SH-SY5Y cell line. Eur J Med Chem. https://doi.org/10.1016/j.ejmech.2015.04.038
Trovato Salinaro A, Pennisi M, Di Paola R et al (2018) Neuroinflammation and neurohormesis in the pathogenesis of Alzheimer’s disease and Alzheimer-linked pathologies: modulation by nutritional mushrooms. Immun Ageing. https://doi.org/10.1186/s12979-017-0108-1
Pastuszko A (1980) Action of barbiturates on activity of acetylcholinesterase from synaptosomal membranes. Neurochem Res. https://doi.org/10.1007/BF00964714
Kakinuma Y, Furihata M, Akiyama T et al (2010) Donepezil, an acetylcholinesterase inhibitor against Alzheimer’s dementia, promotes angiogenesis in an ischemic hindlimb model. J Mol Cell Cardiol. https://doi.org/10.1016/j.yjmcc.2009.11.010
Bartolini M, Bertucci C, Cavrini V, Andrisano V (2003) β-Amyloid aggregation induced by human acetylcholinesterase: inhibition studies. Biochem Pharmacol. https://doi.org/10.1016/S0006-2952(02)01514-9
García-Ayllón M-S (2011) Revisiting the role of acetylcholinesterase in Alzheimer’s disease: cross-talk with P-tau and β-amyloid. Front Mol Neurosci. https://doi.org/10.3389/fnmol.2011.00022
Von Bernhardi R, Alarcón R, Mezzano D et al (2005) Blood cells cholinesterase activity in early stage Alzheimer’s disease and vascular dementia. Dement Geriatr Cogn Disord. https://doi.org/10.1159/000083500
Blennow K (2010) Biomarkers in Alzheimer’s disease drug development. Nat Med. https://doi.org/10.1038/nm.2221
Sánchez JM, Demartini AF, Roca ADTL (2003) Interaction of donepezil and muscular blockers in Alzheimer’s disease. Rev Esp Anestesiol Reanim 50:97–100
Curatola G, Mazzanti L, Lenaz G, Pastuszko A (1979) General anesthetics inhibit erythrocyte acetylcholinesterase only when membrane-bound. Bull Mol Biol Med 4:139–146
Spinedi A, Pacini L, Luly P (2015) A study of the mechanism by which some amphiphilic drugs affect human erythrocyte acetylcholinesterase activity. Biochem J. https://doi.org/10.1042/bj2610569
Cohen ML, Chan SL, Bhargava HN, Trevor AJ (1974) Inhibition of mammalian brain acetylcholinesterase by ketamine. Biochem Pharmacol. https://doi.org/10.1016/0006-2952(74)90377-3
Braswell LM, Kitz RJ (1977) The effect in vitro of volatile anesthetics on the activity of cholinesterases. J Neurochem. https://doi.org/10.1111/j.1471-4159.1977.tb07784.x
Tassonyi E, Charpantier E, Muller D et al (2002) The role of nicotinic acetylcholine receptors in the mechanisms of anesthesia. Brain Res Bull. https://doi.org/10.1016/S0361-9230(01)00740-7
Hertle I, Scheller M, Bufler J et al (1997) Interaction of midazolam with the nicotinic acetylcholine receptor of mouse myotubes. Anesth Analg. https://doi.org/10.1097/00000539-199707000-00031
Acknowledgements
The authors are grateful for the financial which provided by the Research Foundation of Atatürk University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Işık, M. The Binding Mechanisms and Inhibitory Effect of Intravenous Anesthetics on AChE In Vitro and In Vivo: Kinetic Analysis and Molecular Docking. Neurochem Res 44, 2147–2155 (2019). https://doi.org/10.1007/s11064-019-02852-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02852-y