Abstract
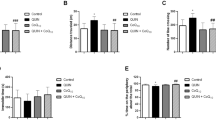
Under pathological conditions, nitric oxide can become a mediator of oxidative cellular damage, generating an unbalance between oxidant and antioxidant systems. The participation of neuronal nitric oxide synthase (nNOS) in the neurodegeneration mechanism has been reported; the activation of N-methyl-d-aspartate (NMDA) receptors by agonist quinolinic acid (QUIN) triggers an increase in nNOS function and promotes oxidative stress. The aim of the present work was to elucidate the participation of nNOS in QUIN-induced oxidative stress in knock-out mice (nNOS−/−). To do so, we microinjected saline solution or QUIN in the striatum of wild-type (nNOS +/+), heterozygote (nNOS+/−), and knock-out (nNOS−/−) mice, and measured circling behavior, GABA content levels, oxidative stress, and NOS expression and activity. We found that the absence of nNOS provides a protection against striatal oxidative damage induced by QUIN, resulting in decreased circling behavior, oxidative stress, and a partial protection reflected in GABA depletion. We have shown that nNOS-derived NO is involved in neurological damage induced by oxidative stress in a QUIN-excitotoxic model.





Similar content being viewed by others
Abbreviations
- CB:
-
Circling behavior
- DCF:
-
Dichlorofluorescein
- DCFH-DA:
-
2’,7’-Dichlorodihydrofluorescein diacetate
- ECL:
-
Enhanced chemiluminescence
- HD:
-
Huntington’s disease
- HPLC:
-
High-performance liquid chromatography
- LP:
-
Lipid peroxidation
- NEO:
-
Neomycin
- NO:
-
Nitric oxide
- nNOS:
-
Neuronal nitric oxide synthase
- OD:
-
Optical density
- QUIN:
-
Quinolinic acid
- ROS:
-
Reactive oxygen species
- SEM:
-
Standard error of the mean
- SS:
-
Saline solution
References
Calabrese V, Mancuso C, Calvani M et al (2007) Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci 8:766–775. https://doi.org/10.1038/nrn2214
Vieira H, Kroemer G (2004) Mitochondria as targets of apoptosis regulation by nitric oxide. IUBMB Life 55:613–616. https://doi.org/10.1080/15216540310001639652
Szydlowska K, Tymianski M (2010) Calcium, ischemia and excitotoxicity. Cell Calcium 47:122–129. https://doi.org/10.1016/J.CECA.2010.01.003
Pérez-Severiano F, Escalante B, Ríos C (1998) Nitric oxide synthase inhibition prevents acute quinolinate-induced striatal neurotoxicity. Neurochem Res 23:1297–1302
Perez-Severiano F, Escalante B, Vergara P et al (2002) Age-dependent changes in nitric oxide synthase activity and protein expression in striata of mice transgenic for the Huntington’s disease mutation. Brain Res 951:36–42. https://doi.org/10.1016/S0006-8993(02)03102-5
Canzoniero LMT, Granzotto A, Turetsky DM et al (2013) nNOS(+) striatal neurons, a subpopulation spared in Huntington’s disease, possess functional NMDA receptors but fail to generate mitochondrial ROS in response to an excitotoxic challenge. Front Physiol 4:112. https://doi.org/10.3389/fphys.2013.00112
Pérez-Severiano F, Montes S, Gerónimo-Olvera C, Segovia J (2013) Study of oxidative damage and antioxidant systems in two Huntington’s disease rodent models. Humana Press, Totowa, pp 177–200
Huang PL, Dawson TM, Bredt DS et al (1993) Targeted disruption of the neuronal nitric oxide synthase gene. Cell 75(7):1273–1286
Martínez-Lazcano JC, Pérez-Severiano F, Escalante B et al (2007) Selective protection against oxidative damage in brain of mice with a targeted disruption of the neuronal nitric oxide synthase gene. J Neurosci Res 85:1391–1402. https://doi.org/10.1002/jnr.21261
Franklin KBJ, Paxinos G (2008) The mouse brain in stereotaxic coordinates. Elsevier, Boston
Petersén A, Chase K, Puschban Z et al (2002) Maintenance of susceptibility to neurodegeneration following intrastriatal injections of quinolinic acid in a new transgenic mouse model of Huntington’s disease. Exp Neurol 175:297–300. https://doi.org/10.1006/exnr.2002.7885
García-Lara L, Pérez-Severiano F, González-Esquivel D et al (2015) Absence of aryl hydrocarbon receptors increases endogenous kynurenic acid levels and protects mouse brain against excitotoxic insult and oxidative stress. J Neurosci Res 93:1423–1433. https://doi.org/10.1002/jnr.23595
Pérez-Neri I, Castro E, Montes S et al (2007) Arginine, citrulline and nitrate concentrations in the cerebrospinal fluid from patients with acute hydrocephalus. J Chromatogr B 851:250–256. https://doi.org/10.1016/j.jchromb.2006.10.047
Triggs WJ, Willmore LJ (1984) In vivo lipid peroxidation in rat brain following intracortical Fe2+ injection. J Neurochem 42:976–980. https://doi.org/10.1111/j.1471-4159.1984.tb12699.x
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Bredt DS, Snyder SH (1990) Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci USA 87:682–685
García-Tovar CG, Luna J, Mena R et al (2002) Dystrophin isoform Dp7l is present in lamellipodia and focal complexes in human astrocytoma cells U-373 MG. Acta Histochem 104:245–254
Ungerstedt U (1971) Striatal dopamine release after amphetamine or nerve degeneration revealed by rotational behaviour. Acta Physiol Scand 82:49–68. https://doi.org/10.1111/j.1365-201X.1971.tb10999.x
Girouard H, Wang G, Gallo EF et al (2009) NMDA receptor activation increases free radical production through nitric oxide and NOX2. J Neurosci 29:2545–2552. https://doi.org/10.1523/JNEUROSCI.0133-09.2009
Cheng A, Wang S, Cai J et al (2003) Nitric oxide acts in a positive feedback loop with BDNF to regulate neural progenitor cell proliferation and differentiation in the mammalian brain. Dev Biol 258(2):319–333
Fritzen S, Schmitt A, Köth K et al (2007) Neuronal nitric oxide synthase (NOS-I) knockout increases the survival rate of neural cells in the hippocampus independently of BDNF. Mol Cell Neurosci 35(2):261–271. https://doi.org/10.1016/j.mcn.2007.02.021
Kolarow R, Kuhlmann CRW, Munsch T et al (2014) BDNF-induced nitric oxide signals in cultured rat hippocampal neurons: time course, mechanism of generation, and effect on neurotrophin secretion. Front Cell Neurosci 8:323. https://doi.org/10.3389/fncel.2014.00323
Dawson VL, Kizushi VM, Huang PL et al (1996) Resistance to neurotoxicity in cortical cultures from neuronal nitric oxide synthase-deficient mice. J Neurosci 76:2479–2487
Ayata C, Ayata G, Hara H et al (1997) Mechanisms of reduced striatal NMDA excitotoxicity in type I nitric oxide synthase knock-out mice. J Neurosci 17:6908–6917
Steinert JR, Chernova T, Forsythe ID (2010) Nitric oxide signaling in brain function, dysfunction, and dementia. Neurosci 16:435–452. https://doi.org/10.1177/1073858410366481
Orrenius S, Zhivotovsky B, Nicotera P (2003) Calcium: regulation of cell death: the calcium–apoptosis link. Nat Rev Mol Cell Biol 4:552–565. https://doi.org/10.1038/nrm1150
Qin Z, Wang Y, Chasea TN (2000) A caspase-3-like protease is involved in NF-kappaB activation induced by stimulation of N-methyl-D-aspartate receptors in rat striatum. Mol Brain Res 80:111–122
Deckel AW, Tang V, Nuttal D et al (2002) Altered neuronal nitric oxide synthase expression contributes to disease progression in Huntington’s disease transgenic mice. Brain Res 939:76–86. https://doi.org/10.1016/S0006-8993(02)02550-7
Heng MY, Detloff PJ, Wang PL et al (2009) In vivo evidence for NMDA receptor-mediated excitotoxicity in a murine genetic model of Huntington disease. J Neurosci 29:3200–3205. https://doi.org/10.1523/JNEUROSCI.5599-08.2009
Li XJ, Sharp AH, Li SH, Dawson TM, Snyder SH, Ross CA (1996) Huntingtin-associated protein (HAP1): discrete neuronal localizations in the brain resemble those of neuronal nitric oxide synthase. Proc Natl Acad Sci USA 93(10):4839–4844
Funding
This work was supported by Conacyt Grant CB-2014 #241911 to F.P-S and was partially supported by Grant FOSISS-2015-2-261721 to L.T-L.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gerónimo-Olvera, C., Tristán-López, L., Martínez-Lazcano, J.C. et al. Striatal Protection in nNOS Knock-Out Mice After Quinolinic Acid-Induced Oxidative Damage. Neurochem Res 44, 421–427 (2019). https://doi.org/10.1007/s11064-018-2688-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-018-2688-3