Abstract
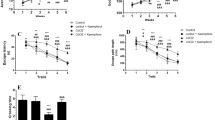
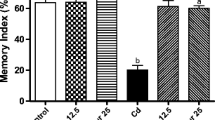
This study investigated if resveratrol (RES) can protect against cadmium chloride (CdCl2)-induced memory loss and Tau protein hyperphosphorylation in rats and explored its effect on AMPK/PI3K/Akt signaling pathway. Rats (n = 10/group) were divided into seven groups as: control; control + DMSO; control + LY294002, a selective PI3K inhibitor (0.25 µg/100 g, i.p); control + RES (300 mg/kg, orally); CdCl2 (5 mg/kg, orally); CdCl2 + RES and CdCl2 + RES + LY294002. All treatments were carried out for 30 consecutive days on a daily basis. RES improved both short and long-term memory as analyzed by novel object recognition task and significantly increased brain levels of glutathione in both control and CdCl2-treated rats. It also inhibited ROS levels of malondialdehyde in the brains of CdCl2-treated rats. In both groups, RES decreased the phosphorylation rate of Tau at Ser199 and Ser296. Concomitantly, it significantly increased protein levels of p-GSK3β (Ser9) and p-PP2A and decreased p-GSK3β (Tyr216). Also, RES activated PI3K/Akt signaling pathway in both control and CdCl2 treated rats by increasing levels of p-PI3K (Tyr607) and p-Akt (Ser473). This was concomitant with significant increase in the levels of AMPK and p-AMPK, known upstream regulators of PI3K/Akt signaling pathway. Interestingly, all the above listed beneficial effects of RES, except their effect on AMPK/p-AMPK, were completely abolished in CdCl2 + RES + LY294002-treated rats. In conclusion, in addition to its antioxidant potential, RES inhibits Tau phosphorylation in rat’s brain by activating PP2A protein and AMPK/PI3K/Akt-induced inhibition of GSK3β.









Similar content being viewed by others
References
Selkoe DJ (2011) Alzheimer’s disease. Cold Spring Harb Perspect Biol 3:1–16
Fath T, Eidenmüller J, Brandt R (2002) Tau-mediated cytotoxicity in a pseudohyperphosphorylation model of Alzheimer’s disease. J Neurosci 2:9733–9741
Zempel H, Luedtke J, Kumar Y, Biernat J, Dawson H, Mandelkow E, Mandelkow EM (2013) Amyloid-β oligomers induce synaptic damage via Tau-dependent microtubule severing by TTLL6 and spastin. EMBO J 32:2920–2937
Swomley AM, Förster S, Keeney JT, Triplett J, Zhang Z, Sultana R, Butterfield DA (2013) A beta, oxidative stress in Alzheimer disease: evidence based on proteomics studies. Biochim Biophys Acta 1842:1248–1257
Alonso AC, Li B, Grundke-Iqbal I, Iqbal K (2008) Mechanism of Tau-induced neurodegeneration in Alzheimer disease and related tauopathies. Curr Alzheimer Res 5:375–384
Landrieu I, Smet-Nocca C, Amniai L, Louis JV, Wieruszeski JM, Goris J, Janssens V, Lippens G (2011) Molecular implication of PP2A and Pin1 in the Alzheimer’s disease specific hyperphosphorylation of Tau. PLoS ONE 6:e21521
Rankin CA, Sun Q, Gamblin TC (2007) Tau phosphorylation by GSK-3β promotes tangle-like filament morphology. Mol Neurodegener 2:12
Ponce-Lopez T, Hong E, Abascal-Díaz M, Meneses A (2017) Role of GSK3β and PP2A on regulation of Tau phosphorylation in hippocampus and memory impairment in ICV-STZ animal model of Alzheimer’s disease. Adv Alzheimer’s Dis 2017:13–31
Lee CW, Lau KF, Miller CC, Shaw PC (2003) Glycogen synthase kinase-3β-mediated Tau phosphorylation in cultured cell lines. Neuroreport 14:257–260
Noh MY, Koh SH, Kim Y, Kim HY, Cho GW, Kim SH (2009) Neuroprotective effects of donepezil through inhibition of GSK-3 activity in amyloid-betainduced neuronal cell death. J Neurochem 108:1116–1125
Mulot S, Hughes K, Woodgett J, Anderton BH, Hanger DP (1994) PHF-Tau from Alzheimer’s brain comprises four species on SDS-PAGE which can be mimicked by in vitro phosphorylation of human brain Tau by glycogen synthase kinase-3b. FEBS Lett 349:359–364
Singh TJ, Zaidi T, Grundke-Iqbal I, Iqbal K (1995) Modulation of GSK-3-catalyzed phosphorylation of microtubule-associated protein Tau by non-proline-dependent protein kinases. FEBS Lett 358:4–8
Iqbal K, Liu F, Gong C (2014) Alzheimer disease therapeutics: focus on the disease and not just plaques and tangles. Biochem Pharmacol 88:631–639
Xiao H, Wang J, Yuan L, Xiao C, Wang Y, Liu X (2013) Chicoric acid induces apoptosis in 3T3-L1 preadipocytes through ROS-mediated PI3K/Akt and MAPK signaling pathways. J Agric Food Chem 61:1509–1520
Zhao R, Zhang Z, Song Y, Wang D, Qi J, Wen S (2011) Implication of phosphatidylinositol-3 kinase/Akt/glycogen synthase kinase-3β pathway in ginsenoside Rb1’s attenuation of beta-amyloid-induced neurotoxicity and Tau phosphorylation. J Ethnopharmacol 133:1109–1116
Min KJ, Lee JT, Joe EH, Kwon TK (2011) An IκBα phosphorylation inhibitor induces hemeoxygenase-1(HO-1) expression through the activation of reactive oxygen species (ROS)-Nrf2-ARE signaling and ROS-PI3K/Akt signaling in an NF-κB-independent mechanism. Cell Signal 23:1505–1513
Hu W, Li F, Mahavadi S, Murthy KS (2009) Upregulation of RGS4 expression by IL-1beta in colonic smooth muscle is enhanced by ERK1/2 and p38 MAPK and inhibited by the PI3K/Akt/GSK3β pathway. Am J Physiol Cell Physiol 296:1310–1320
Yegambaram M, Manivannan B, Beach TG, Halden RU (2015) Role of environmental contaminants in the etiology of Alzheimer’s disease: a review. Curr Alzheimer Res 12:116–146
Wang B, Du Y (2013) Cadmium and its neurotoxic effects. Oxidative medicine and cellular longevity: article ID 898034
Godt J, Scheidig F, Grosse-Siestrup C, Esche V, Brandenburg P, Reich A (2006) The toxicity of cadmium and resulting hazards for human health. J Occup Med Toxicol 1:1–6
Nawrot T, Plusquin M, Hogervorst J, Roels HA, Celis H, Thijs L (2006) Environmental exposure to cadmium and risk of cancer: a prospective populationbased study. Lancet Oncol 7:119–126
Shukla A, Shukla GS, Srimal RC (1996) Cadmium-induced alterations in blood-brain barrier permeability and its possible correlation with decreased microvessel antioxidant potential in rat. Hum Exp Toxicol 15:400–405
Barnham KJ, Bush AI (2008) Metals in Alzheimer’s and Parkinson’s diseases. Curr Opin Chem Biol 12:222–228
Panayi A, Spyrou N, Iversen B, White MA, Part P (2002) Determination of cadmium and zinc in Alzheimer’s brain tissue using inductively coupled plasma mass spectrometry. J Neurol Sci 195:1–10
Jiang LF, Yao TM, Zhu ZL, Wang C, Ji LN (2007) Impacts of Cd (II) on the conformation and self-aggregation of Alzheimer’s Tau fragment corresponding to the third repeat of microtubule-binding domain. Biochim Biophys Acta 1774:1414–1421
Chen L, Xu B, Liu L, Zhou H, Chen W, Shen T, Shen T, Han X, Kontos CD, Huang S (2011) Cadmium induction of reactive oxygen species activates the mTOR pathway, leading to neuronal cell death. Free Radic Biol Med 50:624–632
Goncalves JF, Fiorenza AM, Spanevello RM, Mazzanti CM, Bochi GV, Antes FG, Stefanello N, Rubin MA, Dressler VL, Morsch VM, Schetinger MR (2010) N-Acetylcysteine prevents memory deficits, the decrease in acetylcholinesterase activity and oxidative stress in rats exposed to cadmium. Chem Biol Interact 186:53–60
Shukla GS, Srivastava RS, Chandra SV (1988) Glutathione status and cadmium neurotoxicity: studies in discrete brain regions of growing rats. Fundam Appl Toxicol 11:229–235
Wang Y, Fang J, Leonard SS, Rao KM (2004) Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic Biol Med 36:1434–1443
del Pino J, Zeballos G, Anadón MJ, Moyano P, Díaz MJ, García JM (2015) Cadmium-induced cell death of basal forebrain cholinergic neurons mediated by muscarinic M1 receptor blockade, increase in GSK-3b enzyme, b-amyloid and Tau protein levels. Arch Toxicol 1:12
Chen L, Liu L, Huang S (2008) Cadmium activates the mitogen activated protein kinase (MAPK) pathway via induction of reactive oxygen species and inhibition of protein phosphatases 2A and 5. Free Radic Biol Med 45:1035–1044
Chen S, Xu Y, Xu B, Guo M, Zhang Z, Liu L, Ma H, Chen Z, Luo Y, Huang S, Chen L (2011) Ca MKII is involved in cadmium activation of MAPK and mTOR pathways leading to neuronal cell death. J Neurochem 119:1108–1118
Pei JJ, Hugon J (2008) mTOR-dependent signalling in Alzheimer’s disease. J Cell Mol Med 12:2525–2532
Tang Z, Bereczki E, Zhang H, Wang S, Li C, Ji X, Branca RM, Lehtiö J, Guan Z, Filipcik P, Xu S, Winblad B, Pei JJ (2013) Mammalian target of rapamycin (mTor) mediates Tau protein dyshomeostasis implication for Alzheimer disease. J Biol Chem 288:15556–15570
Bastianetto S, Zheng WH, Quirion R (2000) Neuroprotective abilities of resveratrol and other red wine constituents against nitric oxide-related toxicity in cultured hippocampal neurons. Br J Pharmacol 131:711–720
Virgili M, Contestabile A (2000) Partial neuroprotection of in vivo excitotoxic brain damage by chronic administration of the red wine antioxidant agent, trans-resveratrol in rats. Neurosci Lett 281:123–126
Nicolini G, Rigolio R, Miloso M, Bertelli AA, Tredici G (2001) Anti-apoptotic effect of trans-resveratrol on paclitaxel-induced apoptosis in the human neuroblastoma SH-SY5Y cell line. Neurosci Lett 302:41–44
Leibovici D, Ritchie K, Ledésert B, Touchon J (1999) The effects of wine and tobacco consumption on cognitive performance in the elderly: a longitudinal study of relative risk. Int J Epidemiol 28:77–81
Orgogozo JM, Dartigues JF, Lafont S, Letenneur L, Commenges D, Salamon R, Renaud S, Breteler MB (1997) Wine consumption and dementia in the elderly: A prospective community study in the. Bordx Rev Neurol 153:185–192
Rege SD, Geetha T, Griffin GD, Broderick TL, Babu J (2014) Neuroprotective effects of resveratrol in Alzheimer disease pathology. Front Aging Neurosci 11:218
Karuppagounder SS, Pinto JT, Xu H (2009) Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochem Int 54:111–118
Porquet D, Grinan-Ferre C, Ferrer I, Camins A, Sanfeliu C, Del Valle J (2014) Neuroprotective role of trans-resveratrol in a murine model of familial Alzheimer’s disease. J Alzheimers Dis 42:1209–1220
Frozza RL, Bernardi A, Hoppe JB, Meneghetti AB, Matté A, Battastini AM, Pohlmann AR, Guterres SS, Salbego C (2013) Neuroprotective effects of resveratrol against Aβ administration in rats are improved by lipid-core nanocapsules. Mol Neurobiol 47:1066–1080
Lin F, Peng Y, Yang QH, MI XJ (2015) Resveratrol inhibits cadmium induced neuronal apoptosis by modulating calcium signalling pathway via regulation of MAPK/mTOR network. Bangladesh J Pharmacol 10:366–376
Liu C, Zhang R, Sun C, Zhang H, Xu C, Liu W et al (2015) Resveratrol prevents cadmium activation of Erk1/2 and JNK pathways from neuronal cell death via protein phosphatases 2A and 5. J Neurochem 135:466–478
Schweiger S, Matthes F, Posey K, Kickstein E, Weber S, Hettich MM et al (2017) Resveratrol induces dephosphorylation of Tau by interfering with the MID1-PP2A complex. Sci Rep 7:13753
Zamin LL, Dillenburg-Pilla P, Argenta-Comiran R, Horn AP, Simão F, Nassif M, Gerhardt D, Frozza RL, Salbego C (2006) Protective effect of resveratrol against oxygen-glucose deprivation in organotypic hippocampal slice cultures: Involvement of PI3-K pathway. Neurobiol Dis 24:170–182
Abdel-Aleem GA, Khaleel EF (2017) Rutin hydrate ameliorates cadmium chloride-induced spatial memory loss and neural apoptosis in rats by enhancing levels of acetylcholine, inhibiting JNK and ERK1/2 activation and activating mTOR signalling, Arch Physiol Biochem 124:367–377
Xiaobin L, Zhonghua QI, Zhao L, YU Z (2016) Astaxanthin reduces type 2 diabeticassociated cognitive decline in rats via activation of PI3K/Akt and attenuation of oxidative stress. Mol Med Rep 13:973–979
Bevins RA, Besheer J (2006) Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat Protoc 1:1306–1311
Zhang Y, Xu Z, Wang H, Dong Y, Shi HN, Culley DJ (2012) Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann Neurol 71(5):687–698
Gil HW, Kang EJ, Lee KH, Yang JO, Lee EY, Hong SY (2011) Effect of glutathione on the cadmium chelation of EDTA in a patient with cadmium intoxication. Hum Exp Toxicol 30:79–83
Mahdavi S, Khodarahmi P, Roodbari NH (2018) Effects of cadmium on Bcl-2/Bax expression ratio in rat cortex brain and hippocampus. Hum Exp Toxicol 37:321–328
Yuan L, Wang J, Xiao H, Xiao C, Wang Y, Liu X (2012) Isoorientin induces apoptosis through mitochondrial dysfunction and inhibition of PI3K/Akt signaling pathway in HepG2 cancer cells. Toxicol Appl Pharmacol 265:83–92
Tsuruta F, Masuyama N, Gotoh Y (2002) The phosphatidylinositol 3-kinase (PI3K)-Akt pathway suppresses Bax translocation to mitochondria. J Biol Chem 277:14040–14047
Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC (1998) Regulation of cell death protease caspase-9 by phosphorylation. Science 282:1318–1321
Lien EC, Lyssiotis CA, Juvekar A, Hu H, Asara JM, Cantley LC et al (2016) Glutathione biosynthesis is a metabolic vulnerability in PI3K/Akt-driven breast cancer. Nat Cell Biol 18:572–578
Das S (2005) Resveratrol-mediated activation of cAMP response element-binding protein through adenosine A3 receptor by Akt-dependent and -independent pathways. J Pharmacol Exp Ther 314:762–769
Crews L, Masliah E (2010) Molecular mechanisms of neurodegeneration in Alzheimer’s disease. Hum Mol Genet 15:12–20
Thatcher RW, Lester ML, McAlaster R, Horst R (1982) Effects of low levels of cadmium and lead on cognitive functioning in children. Arch Environ Health 37:159–166
Marlowe M, Errera J, Jacobs J (1983) Increased lead and cadmium burdens among mentally retarded children and children with borderline intelligence. Am J Ment Defic 87:477–483
Nagymajtenyi DL, Schulz H (1998) Behavioural and neurotoxicological changes caused by cadmium treatment of rats during development. J Appl Toxicol 18:63–70
Akinyem AJ, Oboh J, Fadaka AO (2017) Curcumin administration suppress acetylcholinesterase gene expression in cadmium treated rats. Neurotoxicology 62:75–79
Carageorgiou H, Tzotzes V, Sideris A, Zarros A, Tsakiris S (2005) Cadmium effects on brain acetylcholinesterase activity and antioxidant status of adult rats: modulation by zinc, calcium and L-cysteine co-administration. Basic Clin Pharmacol Toxicol 97:320–324
Blokland A (1996) Acetylcholine: a neurotransmitter for learning and memory. Brain Res Rev 21:285–300
Pari L, Murugavel P (2007) Diallyl tetrasulfide improves cadmium induced alterations of acetylcholinesterase, ATPases and oxidative stress in brain of rats. Toxicology 234:44–50
Heo HJ, Kim MJ, Lee JM, Choi SJ, Cho HY, Hong B, Kim HK, Kim E, Shin DH (2004) Naringenin from Citrus junos has an inhibitory effect on acetylcholinesterase and as mitigating effect on amnesia. Dement Geriatr Cogn Disord 17:151–157
Tota S, Awasthi H, Kamat PK, Nath C, Hanif K (2010) Protective effect of quercetin against intracerebral streptozotocin induced reduction in cerebral blood flow and impairment of memory in mice. Behav Brain Res 209:73–79
Liu SJ, Zhang JY, Li HL, Nath C, Hanif K (2004) Tau becomes a more favorable substrate for GSK-3 when it is prephosphorylated by PKA in rat brain. J Biol Chem 279:50078–50088
Ben P, Zhang Z, Zhu Y, Xiong A, Gao Y, Mu J, Yin Z, Luo L (2016) L-Theanine attenuates cadmium-induced neurotoxicity through the inhibition of oxidative damage and Tau hyperphosphorylation. Neurotoxicology 57:95–103
Shi HR, Zhu LQ, Wang SH, Liu XA, Tian Q, Zhang Q, Wang Q, Wang JZ (2008) 17β-estradiol attenuates glycogen synthase kinase-3β activation and Tau hyperphosphorylation in Akt-independent manner. J Neural Transm 115:79–888
He J, Yamada K, Zou LB, Nabeshima T (2001) Spatial memory deficit and neurodegeneration induced by the direct injection of okadaic acid into the hippocampus in rats. J Neural Transm 108:1435–1443
Wang SH, Shih YL, Kuo TC, Shih CM (2009) Cadmium toxicity toward autophagy through ROS-activated GSK-3b in mesangial cells. Toxicol Sci 108:124–131
Wang JZ, Grundke-Iqbal I, Iqbal K (2007) Kinases and phosphatases and Tau sites involved in Alzheimer neurofibrillary degeneration. Eur J Neurosci 25:59
Moule SK, Welsh GI, Edgell NJ, Foulstone EJ, Proud CG, Denton RM (1997) Regulation of protein kinase B and glycogen synthase kinase-3 by insulin and beta-adrenergic agonists in rat epididymal fat cells. Activation of protein kinase B by wortmannin-sensitive and -insensitive mechanisms. J Biol Chem 272:7713–7719
Persad S, Attwell S, Gray V, Mawji N, Deng JT, Leung D et al (2001) Regulation of protein kinase B/Akt-serine 473 by integrin-linked kinase: critical roles for kinase activity and amino acids arginine 211 and serine 343. J Biol Chem 276:27462–27469
Chen S, Xiao X, Feng X, Li W, Zhou N, Zheng L, Sun Y, Zhang Z, Zhu W (2012) Resveratrol induces Sirt1-dependent apoptosis in 3T3-L1 preadipocytes by activating AMPK and suppressing AKT activity and survivin expression. J Nutr Biochem 23(9):1100–1112
Yang YJ, Hu L, Xia YP, Jiang CY, Miao C, Yang CQ, Yuan M, Wang L (2016) Resveratrol suppresses glial activation and alleviates trigeminal neuralgia via activation of AMPK. J Neuroinflamm 13(1):84
Tao R, Gong J, Luo X, Zang M, Guo W, Wen R, Luo Z (2010) AMPK exerts dual regulatory effects on the PI3K pathway. J Mol Signal 18:5:1
Domise M, Vingtdeux V (2016) AMPK in neurodegenerative diseases. EXS 107:153–177
Martin G, Frasch (2014) Putative role of AMPK in fetal adaptive brain shut-down: linking metabolism and inflammation in the brain. Front Neurol 5:150
Wang L, Liu BJ, Cao Y, Xu WQ, Sun DS, Li MZ, Shi FX, Li M, Tian Q, Wang JZ, Zhou XW (2018) Deletion of type-2 cannabinoid receptor induces Alzheimer’s disease-like Tau pathology and memory impairment through AMPK/GSK3β pathway. Mol Neurobiol 55:4731–4744
Acknowledgements
The authors would like to thank Mr. Mahmoud Alkhateeb from King Saud University for Health Sciences for his partial contribution to this study. They would like to thank the animal facility staff at King Khalid University for taking of animals during the experimental procedure.
Funding
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through General Research Project under grant number (201/39).
Author information
Authors and Affiliations
Contributions
Both AS and MA contributed equally to this work. AS and MA designed the experimental procedure and supervised the treatment and sample collections. MA performed the behavioral analysis and measured some of the biochemical parameters of this study. AS performed all the western blotting measurements. AS and MA collected the data, analyzed them and graphed the data. AS wrote the draft of the manuscript and MA finalized the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shati, A.A., Alfaifi, M.Y. Trans-resveratrol Inhibits Tau Phosphorylation in the Brains of Control and Cadmium Chloride-Treated Rats by Activating PP2A and PI3K/Akt Induced-Inhibition of GSK3β. Neurochem Res 44, 357–373 (2019). https://doi.org/10.1007/s11064-018-2683-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-018-2683-8