Abstract
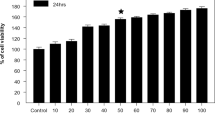
Lead (Pb2+) is a well-known type of neurotoxin and chronic exposure to Pb2+ induces cognition dysfunction. In this work, the potential role of early growth response gene 1 (EGR1) in the linkage of Pb2+ exposure and disrupted in scherophernia-1 (DISC1) activity was investigated. Human neuroblastoma cell line SH-SY5Y was subjected to different concentrations of lead acetate (PbAc) to determine the effect of Pb2+ exposure on the cell viability, apoptosis, and activity of EGR1 and DISC1. Then the expression of EGR1 in SH-SY5Y cells was knocked down with specific siRNA to assess the function of EGR1 in Pb2+ induced activation of DISC1. The interaction between EGR1 and DISC1 was further validated with dual luciferase assay, Supershift electrophoretic mobility shift assay (EMSA), and chromatin immunoprecipitation (ChIP)-PCR. Administration of PbAc decreased cell viability and induced apoptosis in SH-SY5Y cells in a dose-dependent manner. Additionally, exposure to PbAc also up-regulated expression of EGR1 and DISC1 at all concentrations. Knockdown of EGR1 blocked the effect of PbAc on SH-SY5Y cells, indicating the central role of EGR1 in the function of Pb2+ on activity of DISC1. Based on the results of dual luciferase assay, Supershift EMSA, and ChIP-PCR, EGR1 mediated the effect of Pb2+ on DISC1 by directly bound to the promoter region of DISC1 gene. The current study elaborated the mechanism involved in the effect of Pb2+ exposure on expression of DISC1 for the first time: EGR1 activated by Pb2+ substitution of zinc triggered the transcription of DISC1 gene by directly binding to its promoter.



Similar content being viewed by others
Data Accessibility
The supporting data and materials reported in this manuscript are archived in the authors’ personal files. Researchers interested in accessing them should contact YYY or LGS.
References
Ordemann JM, Austin RN (2016) Lead neurotoxicity: exploring the potential impact of lead substitution in zinc-finger proteins on mental health. Metallomics 8:579–588
Krapivinsky G, Krapivinsky L, Manasian Y et al (2003) The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron 40:775–784
Toscano CD, Guilarte TR (2005) Lead neurotoxicity: from exposure to molecular effects. Brain Res Rev 49:529
Lidsky TI, Schneider JS (2003) Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain 126:5–19
Feldman RG, White RF (1992) Lead neurotoxicity and disorders of learning. J Child Neurol 7:354–359
Patrick L (2006) Lead toxicity, a review of the literature. Part 1: exposure, evaluation, and treatment. Altern Med Rev A J Clin Ther 11:2–22
Gellert GA, Wagner GA, Maxwell RM, Moore D, Foster L (1994) Lead poisoning: from screening to primary prevention. Pediatrics 93:343
Bakulski KM, Rozek LS, Dolinoy DC, Paulson HL, Hu H (2012) Alzheimer’s disease and environmental exposure to lead: the epidemiologic evidence and potential role of epigenetics. Curr Alzheimer Res 9:563–573
Lee J, Freeman JL (2014) Zebrafish as a model for investigating developmental lead (Pb) neurotoxicity as a risk factor in adult neurodegenerative disease: a mini-review. Neurotoxicology 43:57–64
Weisskopf MG, Weuve J, Nie HL et al (2010) Association of cumulative lead exposure with Parkinson’s disease. Environ Health Perspect 118:1609
Coon S, Stark A, Peterson E et al (2006) Whole-body lifetime occupational lead exposure and risk of Parkinson’s disease. Environ Health Perspect 114:1872–1876
Opler MGA, Brown AS, Graziano J et al (2004) Prenatal lead exposure, delta-aminolevulinic acid, and schizophrenia. Environ Health Perspect 112:548–552
Guilarte TR, Opler M, Pletnikov M (2012) Is lead exposure in early life an environmental risk factor for Schizophrenia? Neurobiological connections and testable hypotheses. Neurotoxicology 33:560–574
Ahn Y, Yoon JH (2015) P202 The association between blood lead level and clinical mental disorders in fifty thousand lead-exposed male workers. J Affect Disord 190:41
Sadiq S, Ghazala Z, Chowdhury A, Büsselberg D (2012) Metal toxicity at the synapse: presynaptic, postsynaptic, and long-term effects. J Toxicol. https://doi.org/10.1155/2012/132671
Coyle JT, Tsai G, Goff D (2003) Converging evidence of NMDA receptor hypofunction in the pathophysiology of Schizophrenia. Ann N Y Acad Sci 1003:318–327
Robertson HA (1992) Dopamine receptor interactions: some implications for the treatment of Parkinson’s disease. Trends Neurosci 15:201–206
Ming GL, Song H (2009) DISC1 partners with GSK3β in neurogenesis. Cell 136:990–992
Millar JK, Wilson-Annan JC, Anderson S et al (2000) Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet 9:1415
Blackwood DH, Fordyce A, Walker MT, St Clair DM, Porteous DJ, Muir WJ (2001) Schizophrenia and affective disorders–cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family. Am J Hum Genet 69:428
Hamshere ML, Bennett P, Williams N et al (2005) Genomewide linkage scan in schizoaffective disorder: significant evidence for linkage at 1q42 close to DISC1, and suggestive evidence at 22q11 and 19p13. Arch Gen Psychiatry 62:1081–1088
Hashimoto R, Numakawa T, Ohnishi T et al (2006) Impact of the DISC1 Ser704Cys polymorphism on risk for major depression, brain morphology and ERK signaling. Hum Mol Genet 15:3024
Ju YK, Duan X, Liu CY et al (2009) DISC1 regulates new neuron development in the adult brain via modulation of AKT-mTOR signaling through KIAA1212. Neuron 63:761
Thiel G, Cibelli G (2002) Regulation of life and death by the zinc finger transcription factor Egr-1. J Cell Physiol 193:287
Smith TS, Trimmer PA, Khan SM, Tinklepaugh DL, Bennett JP Jr (1997) Mitochondrial toxins in models of neurodegenerative diseases. II: Elevated zif268 transcription and independent temporal regulation of striatal D1 and D2 receptor mRNAs and D1 and D2 receptor-binding sites in C57BL/6 mice during MPTP treatment. Brain Res 765:189
Tureyen K, Brooks N, Bowen K, Svaren J, Vemuganti R (2008) Transcription factor early growth response-1 induction mediates inflammatory gene expression and brain damage following transient focal ischemia. J Neurochem 105:1313–1324
Reddy GR, Zawia NH (2000) Lead exposure alters Egr-1 DNA-binding in the neonatal rat brain. Int J Dev Neurosci 18:791–795
Zawia NH, Harry GJ (1995) Exposure to lead-acetate modulates the developmental expression of myelin genes in the rat frontal lobe. Int J Dev Neurosci 13:639
Zawia NH, Harry GJ (1996) Developmental exposure to lead interferes with glial and neuronal differential gene expression in the rat cerebellum. Toxicol Appl Pharmacol 138:43–47
Schmitt TJ, Zawia N, Harry GJ (1996) GAP-43 mRNA expression in the developing rat brain: alterations following lead-acetate exposure. Neurotoxicology 17:407–414
Basha MR, Wei W, Brydie M, Razmiafshari M, Zawia NH (2003) Lead-induced developmental perturbations in hippocampal Sp1 DNA-binding are prevented by zinc supplementation: in vivo evidence for Pb and Zn competition. Int J Dev Neurosci 21:1–12
Razmiafshari M, Kao J, D’Avignon A, Zawia NH (2001) NMR identification of heavy metal-binding sites in a synthetic zinc finger peptide: toxicological implications for the interactions of xenobiotic metals with zinc finger proteins. Toxicol Appl Pharmacol 172:1
Razmiafshari M, Zawia NH (2000) Utilization of a synthetic peptide as a tool to study the interaction of heavy metals with the zinc finger domain of proteins critical for gene expression in the developing brain. Toxicol Appl Pharmacol 166:1–12
Huang M, Krepkiy D, Hu W, Petering DH: Zn- (2004) Cd-, and Pb-transcription factor IIIA: properties, DNA binding, and comparison with TFIIIA-finger 3 metal complexes. J Inorg Biochem 98:775
Garrick MD, Dolan KG, Horbinski C et al (2003) DMT1: a mammalian transporter for multiple metals. Biometals 16:41–54
Knapska E, Kaczmarek L (2004) A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Prog Neurobiol 74:183–211
Khachigian LM, Williams AJ, Collins T (1995) Interplay of Sp1 and Egr-1 in the proximal platelet-derived growth factor A-chain promoter in cultured vascular endothelial cells. J Biol Chem 270:27679–27686
Khachigian LM, Lindner V, Williams AJ, Collins T (1996) Egr-1-induced endothelial gene expression: a common theme in vascular injury. Science 271:1427–1431
Svaren J, Ehrig T, Abdulkadir SA, Ehrengruber MU, Watson MA, Milbrandt J (2000) EGR1 target genes in prostate carcinoma cells identified by microarray analysis. J Biol Chem 275:38524
Townsend KJ, Zhou P, Qian L et al (1999) Regulation of MCL1 through a serum response factor/Elk-1-mediated mechanism links expression of a viability-promoting member of the BCL2 family to the induction of hematopoietic cell differentiation. J Biol Chem 274:1801–1813
Tang KF, Song GB, Shi YS, Yuan L, Li YH (2010) Dicer knockdown induces fibronectin-1 expression in HEK293T cells via induction of Egr1. Biochim et Biophys Acta 1800:380
Baron V, Adamson ED, Calogero A, Ragona G, Mercola D (2006) The transcription factor Egr1 is a direct regulator of multiple tumor suppressors including TGFbeta1, PTEN, p53, and fibronectin. Cancer Gene Ther 13:115–124
Arora S, Wang Y, Jia Z et al (2008) Egr1 regulates the coordinated expression of numerous EGF receptor target genes as identified by ChIP-on-chip. Genome Biol 9:R166
Bradshaw NJ, Soares DC, Carlyle BC et al (2011) PKA phosphorylation of NDE1 is DISC1/PDE4 dependent and modulates its interaction with LIS1 and NDEL1. J Neurosci 31:9043
Ekelund J, Lichtermann D, Hovatta I et al (2000) Genome-wide scan for schizophrenia in the Finnish population: evidence for a locus on chromosome 7q22. Hum Mol Genet 9:1049–1057
Unda BK, Kwan V, Singh KK (2016) Neuregulin-1 regulates cortical inhibitory neuron dendrite and synapse growth through DISC1. Neural Plast. https://doi.org/10.1155/2016/7694385
Kang E, Kim JY, Liu CY et al. (2015) Rheb1 mediates DISC1-dependent regulation of new neuron development in the adult hippocampus. Neurogenesis 2:e1081715
Lepagnol-Bestel AM, Kvajo M, Karayiorgou M, Simonneau M, Gogos JA (2013) A Disc1 mutation differentially affects neurites and spines in hippocampal and cortical neurons. Mol Cell Neurosci 54:84–92
Author information
Authors and Affiliations
Contributions
SBB provided the predictive information; YYY and BP designed and conducted the experiments, YYY and SBB collected the data, WJH and WJ provided instruction during experiments; YYY and LGS analysis data separately; YYY completed the draft of manuscript; LGS revised manuscript draft; all authors confirmed the version before submitting.
Corresponding author
Ethics declarations
Conflict of interest
Authors declared no conflict of interest.
Rights and permissions
About this article
Cite this article
You, Y., Peng, B., Ben, S. et al. Lead Neurotoxicity on Human Neuroblastoma Cell Line SH-SY5Y is Mediated via Transcription Factor EGR1/Zif268 Induced Disrupted in Scherophernia-1 Activation. Neurochem Res 43, 1308–1316 (2018). https://doi.org/10.1007/s11064-018-2539-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-018-2539-2