Abstract
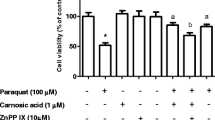
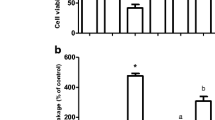
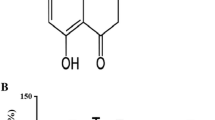
Naringenin (NGN; 5,7-dihydroxy-2-(4-hydroxyphenyl)-2,3-dihydrochromen-4-one; C15H12O5), a flavanone, is found in citrus fruits and has been viewed as an antioxidant and anti-inflammatory agent. NGN is a potent inducer of the nuclear factor erythroid 2-related factor 2 (Nrf2) and upregulates the expression of heme oxygenase-1 (HO-1), an enzyme exhibiting both antioxidant and anti-inflammatory effects. The complete mechanism by which NGN exerts anti-inflammatory actions is not completely understood yet. Therefore, we investigated here whether NGN would be able to reduce the inflammation induced by paraquat (PQ) in SH-SY5Y cells. Additionally, we analyzed the mechanism associated with the NGN-induced anti-inflammatory effect. We found that a pretreatment with NGN at 80 µM for 2 h decreased the levels of pro-inflammatory cytokines interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) in PQ-treated SH-SY5Y cells. The production of nitric oxide (NO·) and levels of cyclooxygenase-2 (COX-2) and of the inducible isoform of nitric oxide synthase (iNOS) were downregulated by NGN in the cells exposed to PQ. Moreover, NGN downregulated the activation of the nuclear factor-κB (NF-κB) in PQ-treated cells. The anti-apoptotic and anti-inflammatory effects promoted by NGN were abolished by ZnPP IX (a specific inhibitor of HO-1) or by knockdown of Nrf2 by small interfering RNA (siRNA). Therefore, NGN induced anti-inflammatory effects in PQ-treated SH-SY5Y cells by a mechanism associated with the Nrf2/HO-1 signaling pathway.









Similar content being viewed by others
References
van der Heide D, Kastelijn J, Schröder-van der Elst JP (2003) Flavonoids and thyroid disease. Biofactors 19:113–119
Hwang SL, Shih PH, Yen GC (2012) Neuroprotective effects of citrus flavonoids. J Agric Food Chem 60:877–885. https://doi.org/10.1021/jf204452y
Escudero-López B, Calani L, Fernández-Pachón MS, Ortega A, Brighenti F, Crozier A, Del Rio D (2014) Absorption, metabolism, and excretion of fermented orange juice (poly)phenols in rats. Biofactors 40:327–335
Esmaeili MA, Alilou M (2014) Naringenin attenuates CCl4 -induced hepatic inflammation by the activation of an Nrf2-mediated pathway in rats. Clin Exp Pharmacol Physiol 41:416–422. https://doi.org/10.1111/1440-1681.12230
Ramprasath T, Senthamizharasi M, Vasudevan V, Sasikumar S, Yuvaraj S, Selvam GS (2014) Naringenin confers protection against oxidative stress through upregulation of Nrf2 target genes in cardiomyoblast cells. J Physiol Biochem 70:407–415. https://doi.org/10.1007/s13105-014-0318-3
Manchope MF, Calixto-Campos C, Coelho-Silva L, Zarpelon AC, Pinho-Ribeiro FA, Georgetti SR, Baracat MM, Casagrande R, Verri WA Jr (2016) Naringenin Inhibits superoxide anion-induced inflammatory pain: role of oxidative stress, cytokines, Nrf-2 and the NO-cGMP-PKG-KATP channel signaling pathway. PLoS ONE 11:e0153015. https://doi.org/10.1371/journal.pone.0153015
Calabrese V, Cornelius C, Mancuso C, Pennisi G, Calafato S, Bellia F, Bates TE, Giuffrida Stella AM, Schapira T, Dinkova Kostova AT, Rizzarelli E (2008) Cellular stress response: a novel target for chemoprevention and nutritional neuroprotection in aging, neurodegenerative disorders and longevity. Neurochem Res 33:2444–2471. https://doi.org/10.1007/s11064-008-9775-9
Lu SC (2013) Glutathione synthesis. Biochim Biophys Acta 1830:3143–3153. https://doi.org/10.1016/j.bbagen.2012.09.008
Nakagami Y (2016) Nrf2 is an attractive therapeutic target for retinal diseases. Oxid Med Cell Longev 2016:7469326
Gullotta F, di Masi A, Coletta M, Ascenzi P (2012) CO metabolism, sensing, and signaling. Biofactors 38:1–13
O’Brien L, Hosick PA, John K, Stec DE, Hinds TD Jr (2015) Biliverdin reductase isozymes in metabolism. Trends Endocrinol Metab 26:212–220. https://doi.org/10.1016/j.tem.2015.02.001
Brown KK, Hampton MB (2011) Biological targets of isothiocyanates. Biochim Biophys Acta 1810:888–894. https://doi.org/10.1016/j.bbagen.2011.06.004
Taki-Nakano N, Ohzeki H, Kotera J, Ohta H (2014) Cytoprotective effects of 12-oxo phytodienoic acid, a plant-derived oxylipin jasmonate, on oxidative stress-induced toxicity in human neuroblastoma SH-SY5Y cells. Biochim Biophys Acta 1840:3413–3422. https://doi.org/10.1016/j.bbagen.2014.09.003
Lou H, Jing X, Wei X, Shi H, Ren D, Zhang X (2014) Naringenin protects against 6-OHDA-induced neurotoxicity via activation of the Nrf2/ARE signaling pathway. Neuropharmacology 79:380–388. https://doi.org/10.1016/j.neuropharm.2013.11.026
Lukiw WJ, Bazan NG (2000) Neuroinflammatory signaling upregulation in Alzheimer’s disease. Neurochem Res 25:1173–1184
Mattson MP (2005) NF-kappaB in the survival and plasticity of neurons. Neurochem Res 30:883–893
Lee CH, Jeon YT, Kim SH, Song YS (2007) NF-kappaB as a potential molecular target for cancer therapy. Biofactors 29:19–35
Shih RH, Wang CY, Yang CM (2015) NF-kappaB signaling pathways in neurological inflammation: a mini review. Front Mol Neurosci 8:77. https://doi.org/10.3389/fnmol.2015.00077
Kim HP, Ryter SW, Choi AM (2006) CO as a cellular signaling molecule. Annu Rev Pharmacol Toxicol 46:411–449
Desmard M, Boczkowski J, Poderoso J, Motterlini R (2007) Mitochondrial and cellular heme-dependent proteins as targets for the bioactive function of the heme oxygenase/carbon monoxide system. Antioxid Redox Signal 9:2139–2155
Tak PP, Firestein GS (2001) NF-kappaB: a key role in inflammatory diseases. J Clin Invest 107:7–11
Hoesel B, Schmid JA (2013) The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer 12:86. https://doi.org/10.1186/1476-4598-12-86
Kay E, Scotland RS, Whiteford JR (2014) Toll-like receptors: role in inflammation and therapeutic potential. Biofactors 40:284–294
Villa V, Thellung S, Bajetto A, Gatta E, Robello M, Novelli F, Tasso B, Tonelli M, Florio T (2016) Novel celecoxib analogues inhibit glial production of prostaglandin E2, nitric oxide, and oxygen radicals reverting the neuroinflammatory responses induced by misfolded prion protein fragment 90–231 or lipopolysaccharide. Pharmacol Res 113:500–514. https://doi.org/10.1016/j.phrs.2016.09.010
Lee JE, Park JH, Jang SJ, Koh HC (2014) Rosiglitazone inhibits chlorpyrifos-induced apoptosis via modulation of the oxidative stress and inflammatory response in SH-SY5Y cells. Toxicol Appl Pharmacol 278:159–171. https://doi.org/10.1016/j.taap.2014.04.021
Park JH, Park YS, Lee JB, Park KH, Paik MK, Jeong M, Koh HC (2016) Meloxicam inhibits fipronil-induced apoptosis via modulation of the oxidative stress and inflammatory response in SH-SY5Y cells. J Appl Toxicol 36:10–23. https://doi.org/10.1002/jat.3136
Aktan F (2004) iNOS-mediated nitric oxide production and its regulation. Life Sci 75:639–653
Arias-Salvatierra D, Silbergeld EK, Acosta-Saavedra LC, Calderon-Aranda ES (2011) Role of nitric oxide produced by iNOS through NF-κB pathway in migration of cerebellar granule neurons induced by Lipopolysaccharide. Cell Signal 23:425–435. https://doi.org/10.1016/j.cellsig.2010.10.017
Dai Z, Wu Z, Yang Y, Wang J, Satterfield MC, Meininger CJ, Bazer FW, Wu G (2013) Nitric oxide and energy metabolism in mammals. Biofactors 39:383–391
Cao H, Yu R, Choi Y, Ma ZZ, Zhang H, Xiang W, Lee DY, Berman BM, Moudgil KD, Fong HH, van Breemen RB (2010) Discovery of cyclooxygenase inhibitors from medicinal plants used to treat inflammation. Pharmacol Res 61:519–524. https://doi.org/10.1016/j.phrs.2010.02.007
Yang W, Tiffany-Castiglioni E, Lee MY, Son IH (2010) Paraquat induces cyclooxygenase-2 (COX-2) implicated toxicity in human neuroblastoma SH-SY5Y cells. Toxicol Lett 199:239–246. https://doi.org/10.1016/j.toxlet.2010.09.005
de Oliveira MR, Ferreira GC, Schuck PF (2016) Protective effect of carnosic acid against paraquat-induced redox impairment and mitochondrial dysfunction in SH-SY5Y cells: role for PI3K/Akt/Nrf2 pathway. Toxicol In Vitro 32:41–54. https://doi.org/10.1016/j.tiv.2015.12.005
de Oliveira MR, Schuck PF, Bosco SM (2016) Tanshinone I induces mitochondrial protection through an Nrf2-dependent mechanism in paraquat-treatedhuman neuroblastoma SH-SY5Y cells. Mol Neurobiol. https://doi.org/10.1007/s12035-016-0009-x
de Oliveira MR, Peres A, Gama CS, Bosco SM (2016) Pinocembrin provides mitochondrial protection by the activation of the Erk1/2-Nrf2 signaling pathway in SH-SY5Y neuroblastoma cells exposed to paraquat. Mol Neurobiol. https://doi.org/10.1007/s12035-016-0135-5
de Oliveira MR, de Souza IC, Fürstenau CR (2017) Carnosic acid induces anti-inflammatory effects in paraquat-treated SH-SY5Y cells through a mechanism involving a crosstalk between the Nrf2/HO-1 Axis and NF-κB. Mol Neurobiol. https://doi.org/10.1007/s12035-017-0389-6
de Oliveira MR, Peres A, Ferreira GC, Schuck PF, Gama CS, Bosco SM (2016) Carnosic acid protects mitochondria of human neuroblastoma SH-SY5Y cells exposed to paraquat through activation of the Nrf2/HO-1Axis. Mol Neurobiol. https://doi.org/10.1007/s12035-016-0100-3
Li F, Tian X, Zhan X, Wang B, Ding M, Pang H (2017) Clathrin-dependent uptake of paraquat into SH-SY5Y cells and its internalization into different subcellular compartments. Neurotox Res 32:204–217. https://doi.org/10.1007/s12640-017-9722-0
Ortiz-Ortiz MA, Morán JM, González-Polo RA, Niso-Santano M, Soler G, Bravo-San Pedro JM, Fuentes JM (2009) Nitric oxide-mediated toxicity in paraquat-exposed SH-SY5Y cells: a protective role of 7-nitroindazole. Neurotox Res 16:160–173. https://doi.org/10.1007/s12640-009-9065-6
Labbé RF, Vreman HJ, Stevenson DK (1999) Zinc protoporphyrin: a metabolite with a mission. Clin Chem 45:2060–2072
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
de Oliveira MR, Peres A, Ferreira GC, Schuck PF, Bosco SM (2016) Carnosic acid affords mitochondrial protection in chlorpyrifos-treated Sh-Sy5y cells. Neurotox Res 30:367–379. https://doi.org/10.1007/s12640-016-9620-x
de Oliveira MR, Ferreira GC, Schuck PF, Dal Bosco SM (2015) Role for the PI3K/Akt/Nrf2 signaling pathway in the protective effects of carnosic acid against methylglyoxal-induced neurotoxicity in SH-SY5Y neuroblastoma cells. Chem Biol Interact 242:396–406. https://doi.org/10.1016/j.cbi.2015.11.003
Scapagnini G, Vasto S, Abraham NG, Caruso C, Zella D, Fabio G (2011) Modulation of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol Neurobiol 44:192–201. https://doi.org/10.1007/s12035-011-8181-5
Rehman MU, Tahir M, Khan AQ, Khan R, Lateef A, Oday-O-Hamiza, Qamar W, Ali F, Sultana S (2013) Chrysin suppresses renal carcinogenesis via amelioration of hyperproliferation, oxidative stress and inflammation: plausible role of NF-κB. Toxicol Lett 216:146–158. https://doi.org/10.1016/j.toxlet.2012.11.013
Sharma N, Nehru B (2015) Characterization of the lipopolysaccharide induced model of Parkinson’s disease: Role of oxidative stress and neuroinflammation. Neurochem Int 87:92–105. https://doi.org/10.1016/j.neuint.2015.06.004
Bhattacharjee N, Borah A (2016) Oxidative stress and mitochondrial dysfunction are the underlying events of dopaminergic neurodegeneration in homocysteine rat model of Parkinson’s disease. Neurochem Int 101:48–55. https://doi.org/10.1016/j.neuint.2016.10.001
Niranjan R (2014) The role of inflammatory and oxidative stress mechanisms in the pathogenesis of Parkinson’s disease: focus on astrocytes. Mol Neurobiol 49:28–38. https://doi.org/10.1007/s12035-013-8483-x
Ljubisavljevic S, Stojanovic I (2015) Neuroinflammation and demyelination from the point of nitrosative stress as a new target for neuroprotection. Rev Neurosci 26:49–73. https://doi.org/10.1515/revneuro-2014-0060
Ljubisavljevic S (2016) Oxidative stress and neurobiology of demyelination. Mol Neurobiol 53:744–758. https://doi.org/10.1007/s12035-014-9041-x
Jarrott B, Williams SJ (2016) Chronic brain inflammation: the neurochemical basis for drugs to reduce inflammation. Neurochem Res 41:523–533. https://doi.org/10.1007/s11064-015-1661-7
Hua FZ, Ying J, Zhang J, Wang XF, Hu YH, Liang YP, Liu Q, Xu GH (2016) Naringenin pre-treatment inhibits neuroapoptosis and ameliorates cognitive impairment in rats exposed to isoflurane anesthesia by regulating the PI3/Akt/PTEN signalling pathway and suppressing NF-κB-mediated inflammation. Int J Mol Med 38:1271–12780. https://doi.org/10.3892/ijmm.2016.2715
Pinho-Ribeiro FA, Zarpelon AC, Fattori V, Manchope MF, Mizokami SS, Casagrande R, Verri WA Jr (2016) Naringenin reduces inflammatory pain in mice. Neuropharmacology 105:508–519. https://doi.org/10.1016/j.neuropharm.2016.02.019
Yang J, Li Q, Zhou XD, Kolosov VP, Perelman JM (2011) Naringenin attenuates mucous hypersecretion by modulating reactive oxygen species production and inhibiting NF-κB activity via EGFR-PI3K-Akt/ERK MAPKinase signaling in human airway epithelial cells. Mol Cell Biochem 351:29–40. https://doi.org/10.1007/s11010-010-0708-y
Acknowledgements
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de Mato Grosso (FAPEMAT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11064_2018_2495_MOESM1_ESM.pdf
Figure S1—The effects of NGN treatment on the Nrf2/HO-1 axis. SH-SY5Y cells were treated with NGN at different concentrations for 8 h (a). The effects of a treatment with NGN at 80 µM for different periods on the levels of Nrf2 in the cell nucleus (b). The effects of NGN at different concentrations for 24 h on the content of HO-1 (c). The effects of Nrf2 knockdown (48 h) on the levels of HO-1 in SH-SY5Y cells treated with NGN at 80 µM for 24 h. * p < 0.05 different from the control group; ** p < 0.05 different from the NGN-treated cells transfected with NC siRNA (PDF 100 KB)
Rights and permissions
About this article
Cite this article
de Oliveira, M.R., Andrade, C.M.B. & Fürstenau, C.R. Naringenin Exerts Anti-inflammatory Effects in Paraquat-Treated SH-SY5Y Cells Through a Mechanism Associated with the Nrf2/HO-1 Axis. Neurochem Res 43, 894–903 (2018). https://doi.org/10.1007/s11064-018-2495-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-018-2495-x