Abstract
Any spinal cord injury carries the potential for persistent disability affecting motor, sensory and autonomic functions. To prevent this outcome, it is highly desirable to block a chain of deleterious reactions developing in the spinal areas immediately around the primary lesion. Thus, early timing of pharmacological neuroprotection should be one major strategy whose impact may be first studied with preclinical models. Using a simple in vitro model of the rat spinal cord it is possible to mimic pathological processes like excitotoxicity that damages neurons because of excessive glutamate receptor activation due to injury, or hypoxic/dysmetabolic insult that preferentially affects glia following vascular dysfunction. While ongoing research is exploring the various components of pathways leading to cell death, current treatment principally relies on the off-label use of riluzole (RLZ) or methylprednisolone sodium succinate (MPSS). The mechanism of action of these drugs is diverse as RLZ targets mainly neurons and MPSS targets glia. Even when applied after a transient excitotoxic stimulus, RLZ can provide effective prevention of secondary excitotoxic damage to premotoneurons, although not to motoneurons that remain very vulnerable. This observation indicates persistent inability to express locomotor activity despite pharmacological treatment conferring some histological protection. MPSS can protect glia from dysmetabolic insult, yet it remains poorly effective to prevent neuronal death. In summary, it appears that these pharmacological agents can produce delayed protection for certain cell types only, and that their combined administration does not provide additional benefit. The search should continue for better, mechanism-based neuroprotective agents.


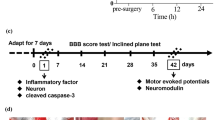
Figures c, d are reprinted from Sámano et al. [46]. A study of the potential neuroprotective effect of riluzole on locomotor networks of the neonatal rat spinal cord in vitro damaged by excitotoxicity. Copyright (2012), with permission from Elsevier

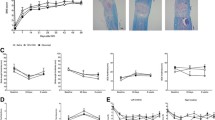
Figures d, e are reprinted from Sámano et al. [56]. A study of methylprednisolone neuroprotection against acute injury to the rat spinal cord in vitro. Copyright (2016), with permission from Elsevier. (Color figure online)

Similar content being viewed by others
Abbreviations
- ALS:
-
Amyotrophic lateral sclerosis
- AMPA:
-
Alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
- ASIA:
-
American Spinal Injury Association
- AU:
-
Arbitrary units
- bcl-xL :
-
Anti-apoptotic regulator, and splicing isoform of bcl-x gene
- BCSFB:
-
Blood-cerebrospinal fluid barrier
- BLMB:
-
Blood-leptomeningeal barrier
- BSB:
-
Blood-spinal barrier
- CD200L:
-
CD200 ligand
- CD200R:
-
CD200 receptor
- CNS:
-
Central nervous system
- CPG:
-
Central pattern generators
- SCI:
-
Spinal cord injury
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- EAAT:
-
Excitatory amino acid transporters
- EPO:
-
Cytokine erythropoietin
- FDA:
-
Food and Drug Administration
- GFAP:
-
Glial fibrillary acidic protein
- GR:
-
Glucocorticoid receptor
- HIF1α:
-
Hypoxic inducing factor 1α
- hCNS-SCns:
-
Human central nervous system-derived neural stem cell
- i.v.:
-
Intravenous
- IL:
-
Interleukin
- KA:
-
Kainate
- LWM:
-
Lateral white matter
- MBP:
-
Mature myelin basic protein
- MPSS:
-
Methylprednisolone sodium succinate
- NASCIS:
-
National Acute Spinal Cord Injury Study Trials
- NMDA:
-
N-methyl-d-aspartic acid
- nNOS:
-
Neuronal nitric oxide synthase
- OLs:
-
Oligodendrocytes
- PM:
-
Pathological medium
- RISCIS:
-
Riluzole in Acute Spinal Cord Injury Study
- RLZ:
-
Riluzole or rilutek
- RNS:
-
Reactive nitrogen species
- ROS:
-
Reactive oxygen species
- SMI-32:
-
Neurofilament H non-phosphorylated antibody
- SOD-1:
-
Superoxide dismutase
- STAT5:
-
Activator transcription factor STAT
- TBOA:
-
Threo-β-benzyloxyaspartate
- TNF-α:
-
Tumor necrosis factor alpha
- TRPM:
-
Transient receptor potential cation channel, subfamily M
- VWM:
-
Ventral white matter
- WM:
-
White matter
- Wsh:
-
Washout
- 5-HT:
-
5-Hydroxytryptamine
References
Furlan JC, Sakakibara BM, Miller WC, Krassioukov AV (2013) Global incidence and prevalence of traumatic spinal cord injury. Can J Neurol Sci 40:456–464
Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG (2014) Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol 6:309–331. https://doi.org/10.2147/CLEP.S68889
Leonard M, Sproule J, McCormack D (2007) Paediatric spinal trauma and associated injuries. Injury 38:188–193
Cristante AF, Barros Filho TE, Marcon RM, Letaif OB, Rocha ID (2002) Therapeutic approaches for spinal cord injury. Clinics 67:1219–1224
Ahuja CS, Martin AR, Fehlings M (2016) Recent advances in managing a spinal cord injury secondary to trauma. F1000Research 5:1–13. https://doi.org/10.12688/f1000research.7586.1
Ahuja CS, Nori S, Tetreault L, Wilson J, Kwon B, Harrop J, Choi D, Fehlings MG (2017) Traumatic spinal cord injury-repair and regeneration. Neurosurgery 1:S9–S22. https://doi.org/10.1093/neuros/nyw080
Rabchevsky AG, Fugaccia I, Sullivan PG, Blades DA, Scheff SW (2002) Efficacy of methylprednisolone therapy for the injured rat spinal cord. J Neurosci Res 1:7–18
Hawryluk GW, Rowland J, Kwon BK, Fehlings MG (2008) Protection and repair of the injured spinal cord: a review of completed, ongoing, and planned clinical trials for acute spinal cord injury. Neurosurg Focus 25:E14. https://doi.org/10.3171/FOC.2008.25.11.E14
Wilson JR, Fehlings MG (2014) Riluzole for acute traumatic spinal cord injury: a promising neuroprotective treatment strategy. World Neurosurg 81:825–829. https://doi.org/10.1016/j.wneu.2013.01.001
Nagoshi N, Nakashima H, Fehlings MG (2015) Riluzole as a neuroprotective drug for spinal cord injury: from bench to bedside. Molecules 20:7775–7789. https://doi.org/10.3390/molecules20057775
Taccola G, Margaryan G, Mladinic M, Nistri A (2008) Kainate and metabolic perturbation mimicking spinal injury differentially contribute to early damage of locomotor networks in the in vitro neonatal rat spinal cord. Neuroscience 155:538–555. https://doi.org/10.1016/j.neuroscience.2008.06.008
Kuzhandaivel A, Nistri A, Mazzone GL, Mladinic M (2011) Molecular mechanisms underlying cell death in spinal networks in relation to locomotor activity after acute injury in vitro. Front Cell Neurosci 5:1–17. https://doi.org/10.3389/fncel.2011.00009
Chang HH, Michaelis EK, Roy S (1984) Functional characteristics of L-glutamate. N-methyl-D-aspartate and kainate receptors in isolated brain synaptic membranes. Neurochem Res 9:903–915
Rossignol S, Schwab M, Schwartz M, Fehlings MG (2007) Spinal cord injury: time to move? J Neurosci 27:11782–11792
van den Berg ME, Castellote JM, Mahillo-Fernandez I, de Pedro-Cuesta J (2010) Incidence of spinal cord injury worldwide: a systematic review. Neuroepidemiology 34:184–192. https://doi.org/10.1159/000279335
Courtine G, van den Brand R, Musienko P (2011) Spinal cord injury: time to move. Lancet 4:1896–1998. https://doi.org/10.1016/S0140-6736(11)60711-3
Rowland JW, Hawryluk GW, Kwon B, Fehlings MG (2008) Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus 25:1–17. https://doi.org/10.3171/FOC.2008.25.11.E2
Ufuk T, Ganesh S, Sigurd B (2005) Spine cord injury: an update. Semin Spine Surg 17:73–83
Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT. Ellegala DB, Dumont AS (2001) Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol 24:254–264
Michaelis EK (1998) Molecular biology of glutamate receptors in the central nervous system and their role in excitotoxicity, oxidative stress and aging. Prog Neurobiol 54:369–415
Park E, Velumian AA, Fehlings MG (2004) The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma 21:754–774
Szydlowska K, Tymianski M (2010) Calcium, ischemia and excitotoxicity. Cell Calcium 47:122–129. https://doi.org/10.1016/j.ceca.2010.01.003
Choi DW (1992) Excitotoxic cell death. J Neurobiol 23:1261–1276
King AE, Woodhouse A, Kirkcaldie MT, Vickers JC (2016) Excitotoxicity in ALS: overstimulation, or overreaction? Exp Neurol 1:162–171. https://doi.org/10.1016/j.expneurol.2015.09.019
Aarts MM, Tymianski M (2005) TRPMs and neuronal cell death. Pflügers Arch 451:243–249
Bianchetti E, Mladinic M, Nistri A (2013) Mechanisms underlying cell death in ischemia-like damage to the rat spinal cord in vitro. Cell Death Dis 4:e707. https://doi.org/10.1038/cddis.2013.237
Lewerenz J, Maher P (2015) Chronic glutamate toxicity in neurodegenerative diseases—what is the evidence? Front Neurosci 16:1–20. https://doi.org/10.3389/fnins.2015.00469
Lau A, Tymianski M (2010) Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch 460:525–542
Fern R, Moller T (2000) Rapid ischemic cell death in immature oligodendrocytes: a fatal glutamate release feedback loop. J Neurosci 20:34–42
Buisson A, Choi DW (1995) The inhibitory mGluR agonist, S-4-carboxy-3-hydroxy-phenylglycine selectively attenuates NMDA neurotoxicity and oxygen-glucose deprivation-induced neuronal death. Neuropharmacology 34:1081–1087
Colwell CS, Altemus KL, Levine MS (1996) Metabotropic glutamate receptor activation selectively limits excitotoxic damage in the intact neostriatum. Brain Res 726:223–226
Pizzi M, Consolandi O, Memo M, Spano PF (1996) Activation of multiple metabotropic glutamate receptor subtypes prevents NMDA-induced excitotoxicity in rat hippocampal slices. Eur J Neurosci 8:1516–1521
Rust R, Kaiser J (2017) Insights into the dual role of inflammation after spinal cord injury. J Neurosci 37:4658–4660. https://doi.org/10.1523/JNEUROSCI.0498-17.2017
Mietto BS, Mostacada K, Martinez AM (2015) Neurotrauma and inflammation: CNS and PNS responses. Mediat Inflamm 2015:251204. https://doi.org/10.1155/2015/251204
Arnett HA, Mason J, Marino M, Suzuki K, Matsushima GK, Ting JP (2001) TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci 4:1116–1122
Fehlings MG, Nguyen DH (2010) Immunoglobulin G: a potential treatment to attenuate neuroinflammation following spinal cord injury. J Clin Immunol 1:S109–S112. https://doi.org/10.1007/s10875-010-9404-7
David S, Kroner A (2011) Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci 12:388–399. https://doi.org/10.1038/nrn3053
Piltti KM1, Salazar DL, Uchida N, Cummings BJ, Anderson AJ (2013) Safety of epicenter versus intact parenchyma as a transplantation site for human neural stem cells for spinal cord injury therapy. Stem Cells Transl Med 2:204–216. https://doi.org/10.5966/sctm.2012-0110
Saghazadeh A, Rezaei N (2017) The role of timing in the treatment of spinal cord injury. Biomed Pharmacother 92:128–139. https://doi.org/10.1016/j.biopha.2017.05.048
Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV (2016) Astrocyte scar formation aids central nervous system axon regeneration. Nature 532:195–200. https://doi.org/10.1038/nature17623
Cohen M, Ben-Yehuda H, Porat Z, Raposo C, Gordon S, Schwartz M (2017) Newly formed endothelial cells regulate myeloid cell activity following spinal cord injury via expression of CD200 ligand. J Neurosci 25:972–985. https://doi.org/10.1523/JNEUROSCI.2199-16.2016
Wright GJ, Jones M, Puklavec MJ, Brown MH, Barclay AN (2001) The unusual distribution of the neuronal/lymphoid cell surface CD200 (OX2) glycoprotein is conserved in humans. 102(2):173–179
Constanti A, Nistri A (1976) A comparative study of the effects of glutamate and kainate on the lobster muscle fibre and the frog spinal cord. Br J Pharmacol 57:359–368
Magnuson DS, Trinder TC, Zhang YP, Burke D, Morassutti DJ, Shields CB (1999) Comparing deficits following excitotoxic and contusion injuries in the thoracic and lumbar spinal cord of the adult rat. Exp Neurol 156:191–204
Mazzone GL, Margaryan G, Kuzhandaivel A, Nasrabady SE, Mladinic M, Nistri A (2010) Kainate-induced delayed onset of excitotoxicity with functional loss unrelated to the extent of neuronal damage in the in vitro spinal cord. Neuroscience 168:451–462. https://doi.org/10.1016/j.neuroscience.2010.03.055
Sámano C, Nasrabady SE, Nistri A (2012) A study of the potential neuroprotective effect of riluzole on locomotor networks of the neonatal rat spinal cord in vitro damaged by excitotoxicity. Neuroscience 222:356–365. https://doi.org/10.1016/j.neuroscience.2012.06.064
Kiehn O, Kjaerulff O (1998) Distribution of central pattern generators for rhythmic motor outputs in the spinal cord of limbed vertebrates. Ann N Y Acad Sci 860:110–129
Tolle TR, Berthele A, Zieglgansberger W, Seeburg PH, Wisden W (1993) The differential expression of 16 NMDA and non-NMDA receptor subunits in the rat spinal cord and in periaqueductal gray. J Neurosci 13:5009–5028
Cifra A, Mazzone GL, Nani F, Nistri A, Mladinic M (2012) Postnatal developmental profile of neurons and glia in motor nuclei of the brainstem and spinal cord, and its comparison with organotypic slice cultures. Dev Neurobiol 72:1140–1160. https://doi.org/10.1002/dneu.20991
McTigue DM, Tripathi RB (2008) The life, death, and replacement of oligodendrocytes in the adult CNS. J Neurochem 107:1–19. https://doi.org/10.1111/j.1471-4159.2008.05570.x
Volpe JJ (1997) Brain injury in the premature infant: from pathogenesis to prevention. Brain Dev 19:519–534
Rosenberg PA, Dai W, Gan XD, Ali S, Fu J, Back SA, Sanchez RM, Segal MM, Follett PL, Jensen FE, Volpe JJ (2003) Mature myelin basic protein-expressing oligodendrocytes are insensitive to kainate toxicity. J Neurosci Res 71:237–245
Follett PL, Rosenberg PA, Volpe JJ, Jensen FE (2000) NBQX attenuates excitotoxic injury in developing white matter. J Neurosci 15:9235–9241
Margaryan G, Mladinic M, Mattioli C, Nistri A (2009) Extracellular magnesium enhances the damage to locomotor networks produced by metabolic perturbation mimicking spinal injury in the neonatal rat spinal cord in vitro. Neuroscience 163:669–682. https://doi.org/10.1016/j.neuroscience.2009.07.005
Kuzhandaivel A, Margaryan G, Nistri A, Mladinic M (2010) Extensive glial apoptosis develops early after hypoxic-dysmetabolic insult to the neonatal rat spinal cord in vitro. Neuroscience 169:325–338. https://doi.org/10.1016/j.neuroscience.2010.05.011
Sámano C, Kaur J, Nistri A (2016) A study of methylprednisolone neuroprotection against acute injury to the rat spinal cord in vitro. Neuroscience 315:136–149. https://doi.org/10.1016/j.neuroscience.2015.12.003
Eliasson MJ, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, Pieper A, Wang ZQ, Dawson TM, Snyder SH, Dawson VL (1997) Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med 3:1089–1095
Nasrabady SE, Kuzhandaivel A, Nistri A (2011) Studies of locomotor network neuroprotection by the selective poly(ADP-ribose) polymerase-1 inhibitor PJ-34 against excitotoxic injury to the rat spinal cord in vitro. Eur J Neurosci 33:2216–2227. https://doi.org/10.1111/j.1460-9568.2011.07714.x
Nasrabady SE, Kuzhandaivel A, Akrami A, Bianchetti E, Milanese M, Bonanno G, Nistri A (2012) Unusual increase in lumbar network excitability of the rat spinal cord evoked by the PARP-1 inhibitor PJ-34 through inhibition of glutamate uptake. Neuropharmacology 63:415–426. https://doi.org/10.1016/j.neuropharm.2012.04.014
Mazzone GL, Mladinic M, Nistri A (2013) Excitotoxic cell death induces delayed proliferation of endogenous neuroprogenitor cells in organotypic slice cultures of the rat spinal cord. Cell Death Dis. https://doi.org/10.1038/cddis.2013.431
Mladinic M, Bianchetti E, Dekanic A, Mazzone GL, Nistri A (2014) ATF3 is a novel nuclear marker for migrating ependymal stem cells in the rat spinal cord. Stem Cell Res 12:815–827. https://doi.org/10.1016/j.scr.2014.03.006
Cox A, Varma A, Banik N (2015) Recent advances in the pharmacologic treatment of spinal cord injury. Metab Brain Dis 30:473–482. https://doi.org/10.1007/s11011-014-9547-y
Mazzone GL, Nistri A (2011) Electrochemical detection of endogenous glutamate release from rat spinal cord organotypic slices as a real-time method to monitor excitotoxicity. J Neurosci Methods 15:128–132. https://doi.org/10.1016/j.jneumeth.2011.01.033
Doble A (1999) The role of excitotoxicity in neurodegenerative disease: implications for therapy. Pharmacol Ther 81:163–221
Guidance on the use of Riluzole (Rilutek) for the treatment of Motor Neurone Disease. Technology appraisal guidance Published: 23 January 2001 http://www.nice.org.uk/guidance/ta20
Bellingham MC (2011) A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: what have we learned in the last decade? CNS Neurosci Ther 17:4–31. https://doi.org/10.1111/j.1755-5949.2009.00116.x
Agrawal S, Fehlings M (1997) The effect of the sodium channel blocker QX-314 on recovery after acute spinal cord axonal injury. J Neurotrauma 14:81–88
Urbani A, Belluzzi O (2000) Riluzole inhibits the persistent sodium current in mammalian CNS neurons. Eur J Neurosci 12:3567–3574
Cifra A, Mazzone GL, Nistri A (2013) Riluzole: what it does to spinal and brainstem neurons and how it does it. Neuroscientist 19:137–144. https://doi.org/10.1177/1073858412444932
Lamanauskas N, Nistri A (2008) Riluzole blocks persistent Na+ and Ca2+ currents and modulates release of glutamate via presynaptic NMDA receptors on neonatal rat hypoglossal motoneurons in vitro. Eur J Neurosci 27:2501–2514. https://doi.org/10.1111/j.1460-9568.2008.06211.x
Cifra A, Nani F, Nistri A (2011) Respiratory motoneurons and pathological conditions: lessons from hypoglossal motoneurons challenged by excitotoxic or oxidative stress. Respir Physiol Neurobiol 179:89–96. https://doi.org/10.1016/j.resp.2011.03.017
Mazzone GL, Nistri A (2011) Delayed neuroprotection by riluzole against excitotoxic damage evoked by kainate on rat organotypic spinal cord cultures. Neuroscience 190:318–327. https://doi.org/10.1016/j.neuroscience.2011.06.013
Wilson JR, Fehlings MG (2014) Riluzole for acute traumatic spinal cord injury: a promising neuroprotective treatment strategy. World Neurosurg 8:825–829. https://doi.org/10.1016/j.wneu.2013.01.001
Kuzhandaivel A, Nistri A, Mladinic M (2010) Kainate-mediated excitotoxicity induces neuronal death in the rat spinal cord in vitro via a PARP-1 dependent cell death pathway (Parthanatos). Cell Mol Neurobiol 30:1001–1012. https://doi.org/10.1007/s10571-010-9531-y
Chang G, Guo Y, Jia Y, Duan W, Li B, Yu J, Li C (2010) Protective effect of combination of sulforaphane and riluzole on glutamate-mediated excitotoxicity. Biol Pharm Bull 33:1477–1483
Verhave PS, Jongsma MJ, Van Den Berg RM, Vanwersch RA, Smit AB, Philippens IH (2012) Neuroprotective effects of riluzole in early phase Parkinson’s disease on clinically relevant parameters in the marmoset MPTP model. Neuropharmacology 62:1700–1707. https://doi.org/10.1016/j.neuropharm.2011.11.016
Wang X, Michaelis EK (2010) Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2010.00012
Gou-Fabregas M, Garcera A, Mincheva S, Perez-Garcia MJ, Comella JX, Soler RM (2009) Specific vulnerability of mouse spinal cord motoneurons to membrane depolarization. J Neurochem 110:1842–1854. https://doi.org/10.1111/j.1471-4159.2009.06278.x
Fehlings MG, Kopjar B, Grossman RG (2016) 329 Efficacy and safety of Riluzole in acute spinal cord injury: rationale and design of AOSpine phase III multicenter double-blinded randomized controlled trial (RISCIS). Neurosurgery. https://doi.org/10.1227/01.neu.0000489818.21218.72
Grossman RG, Fehlings MG, Frankowski RF, Burau KD, Chow DS, Tator C, Teng A, Toups EG, Harrop JS, Aarabi B, Shaffrey CI, Johnson MM, Harkema SJ, Boakye M, Guest JD, Wilson JR (2014) A prospective, multicenter, phase I matched-comparison group trial of safety, pharmacokinetics, and preliminary efficacy of riluzole in patients with traumatic spinal cord injury. J Neurotrauma 31:239–255. https://doi.org/10.1089/neu.2013.2969
Nance JR, Golomb MR (2007) Ischemic spinal cord infarction in children without vertebral fracture. Pediatr Neurol 36:209–216
Bracken MB, Shepard MJ, Collins WF et al (1990) A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal cord injury. Results of the second National Acute Spinal Cord Injury Study. N Engl J Med 322:1405–1411
Bracken MB, Shepard MJ, Collins WF et al (1992) Methylprednisolone or naloxone treatment after acute spinal cord injury: I-year follow-up data. Results of the second National Acute Spinal Cord Injury Study, 1992. J Neurosurg 76:23–31
Cheung V, Hoshide R, Bansal V, Kasper E, Chen CC (2015) Methylprednisolone in the management of spinal cord injuries: lessons from randomized, controlled trials. Surg Neurol Int 6:142. https://doi.org/10.4103/2152-7806.163452
Hall ED (2011) Antioxidant therapies for acute spinal cord injury. Neurotherapeutics 8:152–167. https://doi.org/10.1007/s13311-011-0026-4
Miekisiak G, Kloc W, Janusz W, Kaczmarczyk J, Latka D, Zarzycki D (2014) Current use of methylprednisolone for acute spinal cord injury in Poland: survey study. Eur J Orthop Surg Traumatol 24:S269–S273. https://doi.org/10.1007/s00590-014-1422-3
Lee JM, Yan P, Xiao Q, Chen S, Lee KY, Hsu CY, Xu J (2008) Methylprednisolone protects oligodendrocytes but not neurons after spinal cord injury. J Neurosci 28:3141–3149. https://doi.org/10.1523/JNEUROSCI.5547-07.2008
Xu J, Chen S, Chen H, Xiao Q, Hsu CY, Michael D, Bao J (2009) STAT5 mediates antiapoptotic effects of methylprednisolone on oligodendrocytes. J Neurosci 29:2022–2026. https://doi.org/10.1523/JNEUROSCI.2621-08.2009
Sun YY, Wang CY, Hsu MF, Juan SH, Chang CY, Chou CM, Yang LY, Hung KS, Xu J, Lee YH, Hsu CY (2010) Glucocorticoid protection of oligodendrocytes against excitotoxin involving hypoxia-inducible factor-1alpha in a cell-type-specific manner. J Neurosci 30:9621–9630. https://doi.org/10.1523/JNEUROSCI.2295-10.2010
Bracken MB (2012) Steroids for acute spinal cord injury. Cochrane Database Syst Rev 18:1–51. https://doi.org/10.1002/14651858.CD001046.pub2
Harrop JS (2014) Spinal cord injury: debating the efficacy of methylprednisolone. Neurosurgery 61:30–31. https://doi.org/10.1227/NEU.0000000000000391
Fehlings MG, Wilson JR, Cho N (2014) Methylprednisolone for the treatment of acute spinal cord injury: counterpoint. Neurosurgery 1:36–42. https://doi.org/10.1227/NEU.0000000000000412
Sauerland S, Nagelschmidt M, Mallmann P, Neugebauer EA (2000) Risks and benefits of preoperative high dose methylprednisolone in surgical patients: a systematic review. Drug Saf 23:449–461
Ito Y, Sugimoto Y, Tomioka M, Kai N, Tanaka M (2009) Does high dose methylprednisolone sodium succinate really improve neurological status in patient with acute cervical cord injury?: a prospective study about neurological recovery and early complications. Spine 34:2121–2124. https://doi.org/10.1097/BRS.0b013e3181b613c7
Bowers CA, Kundu B, Rosenbluth J, Hawryluk GW (2016) Patients with spinal cord injuries favor administration of methylprednisolone. PLoS ONE. https://doi.org/10.1371/journal.pone.0145991
Mu X, Azbill RD, Springer JE (2000) Riluzole and methylprednisolone combined treatment improves functional recovery in traumatic spinal cord injury. J Neurotrauma 17:773–780
Shabbir A, Bianchetti E, Nistri A (2015) The volatile anesthetic methoxyflurane protects motoneurons against excitotoxicity in an in vitro model of rat spinal cord injury. Neuroscience 285:269–280. https://doi.org/10.1016/j.neuroscience.2014.11.023
Kaur J, Flores Gutiérrez J, Nistri A (2016) Neuroprotective effect of propofol against excitotoxic injury to locomotor networks of the rat spinal cord in vitro. Eur J Neurosci 44:2418–2430. https://doi.org/10.1111/ejn.13353
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sámano, C., Nistri, A. Mechanism of Neuroprotection Against Experimental Spinal Cord Injury by Riluzole or Methylprednisolone. Neurochem Res 44, 200–213 (2019). https://doi.org/10.1007/s11064-017-2459-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2459-6