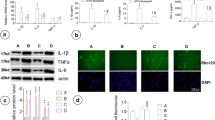
Abstract
The biomolecule acetate can be utilized for energy production, lipid synthesis, and several metabolic processes. Acetate supplementation reduces neuroglial activation in a model of neuroinflammation induced by intraventricular injection of lipopolysaccharide (LPS). To investigate the mechanisms underlying the anti-inflammatory effect of acetate on glial cells, we examined the effect of acetate on nitric oxide (NO) production, which was experimentally activated by LPS, in cultured primary rat astrocytes. Acetate attenuated the LPS-induced NO production in a dose-dependent manner, although cell viability was not affected. Acetate suppressed the phosphorylation of p38-mitogen-activated protein kinase 24 h after LPS treatment. Acetate decreased the LPS-induced production of intracellular reactive oxygen species (ROS) at 4–24 h concomitant with an increase in glutathione. Acetate rescued astrocytes from the hydrogen peroxide-induced cell death by reducing ROS levels. These findings suggest that attenuation of NO production by acetate may alleviate glial cell damage during neuroinflammation. Acetate may offer a glioprotective effect through an anti-oxidative mechanism.





Similar content being viewed by others
Abbreviations
- DAN:
-
2,3-Diaminonaphthalene
- DCF:
-
Dichlorofluorescein
- DMEM:
-
Dulbecco’s modified Eagle medium
- FBS:
-
Fetal bovine serum
- GSH:
-
Glutathione
- H2DCFDA:
-
2′,7′-Dichlorodihydrofluorescein diacetate
- H2O2 :
-
Hydrogen peroxide
- HBS:
-
Hepes-buffered saline
- iNOS:
-
Inducible nitric oxide synthase
- LPS:
-
Lipopolysaccharide
- MAPK:
-
Mitogen-activated protein kinase
- MTT:
-
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-tetrazolium bromide
- NFκB:
-
Nuclear factor-kappaB
- NO:
-
Nitric oxide
- Nrf2:
-
NF-E2-related factor 2
- PBS:
-
Phosphate-buffered saline
- ROS:
-
Reactive oxygen species
References
Comerford SA, Huang Z, Du X, Wang Y, Cai L, Witkiewicz AK, Walters H, Tantawy MN, Fu A, Manning HC, Horton JD, Hammer RE, McKnight SL, Tu BP (2014) Acetate dependence of tumors. Cell 159:1591–1602. doi:10.1016/j.cell.2014.11.020
Moussaieff A, Rouleau M, Kitsberg D, Cohen M, Levy G, Barasch D, Nemirovski A, Shen-Orr S, Laevsky I, Amit M, Bomze D, Elena-Herrmann B, Scherf T, Nissim-Rafinia M, Kempa S, Itskovitz-Eldor J, Meshorer E, Aberdam D, Nahmias Y (2015) Glycolysis-mediated changes in acetyl-CoA and histone acetylation control the early differentiation of embryonic stem cells. Cell Metab 21:392–402. doi:10.1016/j.cmet.2015.02.002
Okabe S, Kodama Y, Cao H, Johannessen H, Zhao CM, Wang TC, Takahashi R, Chen D (2012) Topical application of acetic acid in cytoreduction of gastric cancer. A technical report using mouse model. J Gastroenterol Hepatol 27(Suppl 3):40–48. doi:10.1111/j.1440-1746.2012.07070.x
Long PM, Tighe SW, Driscoll HE, Fortner KA, Viapiano MS, Jaworski DM (2015) Acetate supplementation as a means of inducing glioblastoma stem-like cell growth arrest. J Cell Physiol 230:1929–1943. doi:10.1002/jcp.24927
Anderson CM, Swanson RA (2000) Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia 32:1–14
Correale J, Villa A (2009) Cellular elements of the blood–brain barrier. Neurochem Res 34:2067–2077. doi:10.1007/s11064-009-0081-y
Koehler RC, Gebremedhin D, Harder DR (2006) Role of astrocytes in cerebrovascular regulation. J Appl Physiol 100:307–317
Rossi D, Volterra A (2009) Astrocytic dysfunction: insights on the role in neurodegeneration. Brain Res Bull 80:224–232. doi:10.1016/j.brainresbull.2009.07.012
Ridet JL, Malhotra SK, Pivat A, Gage FH (1997) Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci 20:570–577
Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32:638–647. doi:10.1016/j.tins.2009.08.002
Nomura Y (2001) NF-κB activation and IkBα dynamism involved in iNOS and chemokine induction in astroglial cells. Life Sci 68:1695–1701
Ransohoff RM, Brown MA (2012) Innate immunity in the central nervous system. J Clin Invest 122:1164–1171. doi:10.1172/JCI58644
Saha RN, Pahan K (2006) Signals for the induction of nitric oxide synthase in astrocytes. Neurochem Int 49:154–163
Calabrese V, Mamcuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM (2007) Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci 8:766–775
Murakami K, Nakamura Y, Yoneda Y (2003) Potentiation by ATP of lipopolysaccharide-stimulated nitric oxide production in cultured astrocytes. Neuroscience 117:37–42
Sonnewald U, Kondziella D (2003) Neuronal glia interaction in different neurological diseases studied by ex vivo 13C NMR spectroscopy. NMR Biomed 16:424–429
Waniewski RA, Martin DL (1998) Preferential utilization of acetate by astrocytes is attributable to transport. J Neurosci 18:5225–5233
Sonnewald U, Müller TB, Westergaard N, Unsgård G, Petersen SB, Schouboe A (1994) NMR spectroscopic study of cell cultures of astrocytes and neurons exposed to hypoxia: compartmentation of astrocyte metabolism. Neurochem Int 24:473–483
Sailasuta N, Harris K, Tran T, Ross B (2011) Minimally invasive biomarker confirms glial activation present in Alzheimer’s disease: a preliminary study. Neuropsychiatr Dis Treat 7:495–499
Marik J, Ogasawara A, Martin-McNulty B, Ross J, Flores JE, Gill HS, Tinianow JN, Vanderbilt AN, Nishimura M, Peale F, Pastuskovas C, Greve JM, van Bruggen N, Williams SP (2009) PET of glial metabolism using 2-18F fluoroacetate. J Nucl Med 50:982–990
Reisenauer CJ, Bhatt DP, Mitteness DJ, Slanczka ER, Gienger HM, Watt JA, Rosenberger TA (2011) Acetate supplementation attenuates lipopolysaccharide-induced neuroinflammation. J Neurochem 117:264–274. doi:10.1111/j.1471-4159.2011.07198.x
Arun P, Ariyannur PS, Moffett JR, Xing G, Hamilton K, Grunberg NE, Ives JA, Namboodiri AM (2010) Matabolic acetate therapy for the treatment of traumatic brain injury. J Neurotrauma 27:293–298. doi:10.1007/s10545-010-9100-z
Freyer D, Weih M, Weber JR, Burger W, Scholz P, Manz R, Ziegenhorn A, Angestwurm K, Dirnagl U (1996) Pneumococcal cell wall components induce nitric oxide synthase and TNF-α in astroglial-enriched cultures. Glia 16:1–6
Castano A, Herrera AJ, Cano J, Machado A (1998) Lipopolysaccharide intranigral injection induces inflammatory reaction and damage in nigrostriatal dopaminergic system. J Neurochem 70:1584–1592
Nakamura Y, Kitagawa T, Ihara H, Kozaki S, Moriyama M, Kannan Y (2006) Potentiation by high potassium of lipopolysaccharide-induced nitric oxide production from cultured astrocytes. Neurochem Int 48:43–49
Takano K, Sugita K, Moriyama M, Hashida K, Hibino S, Choshi T, Murakami R, Yamada M, Suzuki H, Hori O, Nakamura Y (2011) A dibenzoylmethane derivative protects against hydrogen peroxide-induced cell death and inhibits lipopolysaccharide-induced nitric oxide production in cultured rat astrocytes. J Neurosci Res 89:955–965. doi:10.1002/jnr.22617
Bhat NR, Feinstein DL, Shen Q, Bhat AN (2002) p38 MAPK-mediated transcriptional activation of inducible nitric-oxide synthase in glial cells. Roles of nuclear factors, nuclear factor kappa B, cAMP response element-binding protein, CCAAT/enhancer-binding protein-beta, and activating transcription factor-2. J Biol Chem 277:29584–29592
Gorina R, Font-Nieves M, Márquez-Kisinousky L, Santalucia T, Planas AM (2011) Astrocyte TLR4 activation induces a proinflammatory environment through the interplay between MyD88-dependent NFκB signaling, MAPK, and Jak1/Stat1 pathways. Glia 59:242–255. doi:10.1002/glia.21094
Lee SY, Son DJ, Lee YK, Lee JW, Lee HJ, Yun YW, Ha TY, Hong JT (2006) Inhibitory effect of sesaminol glucosides on lipopolysaccharide-induced NF-κB activation and target gene expression in cultured rat astrocytes. Neurosci Res 56:204–212
Pawate S, Shen Q, Fan F, Bhat NR (2004) Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J Neurosci Res 77:540–551
Dringen R, Gutterer JM, Hirrlinger J (2000) Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem 267:4912–4916
Czaja MJ, Liu H, Wang Y (2003) Oxidant-induced hepatocyte injury from menadione is regulated by ERK and AP-1 signaling. Hepatology 37:1405–1413
Remacle J, Raes M, Toussaint O, Renard P, Rao G (1995) Low levels of reactive oxygen species as modulators of cell function. Mutat Res 316:103–122
Suzuki YJ, Forman HJ, Sevanian A (1997) Oxidants as stimulators of signal transduction. Free Radic Biol Med 22:269–285
Moriyama M, Jayakumar AR, Tong XY, Norenberg MD (2010) Role of mitogen-activated protein kinases in the mechanism of oxidant-induced cell swelling in cultured astrocytes. J Neurosci Res 88:2450–2458. doi:10.1002/jnr.22400
Hsieh HL, Yang CM (2013) Role of redox signaling in neuroinflammation and neurodegenerative diseases. Biomed Res Int 2013:484613. doi:10.1155/2013/484613.
Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB (2014) Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 20:1126–1167. doi:10.1089/ars.2012.5149
Ozden O, Park SH, Kim HS, Jiang H, Coleman MC, Spitz DR, Gius D (2011) Acetylation of MnSOD directs enzymatic activity responding to cellular nutrient status or oxidative stress. Aging 3:102–107
Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, Johnson JA, Murphy TH (2003) Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci 23:3394–3406
Vargas MR, Pehar M, Cassina P, Beckman JS, Barbeito L (2006) Increased glutathione biosynthesis by Nrf2 activation in astrocytes prevents p75NTR-dependent motor neuron apoptosis. J Neurochem 97:687–696
Sun Z, Chin YE, Zhang DD (2009) Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Mol Cell Biol 29:2658–2672. doi:10.1128/MCB.01639-08
Bhat NR, Zhang P, Lee JC, Hogan EL (1998) Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor a gene expression in endotoxin-stimulated primary glial cultures. J Neurosci 18:1633–1641
Dickinson RJ, Keyse SM (2006) Diverse physiological functions for dual-specificity MAP kinase phosphatases. J Cell Sci 119:4607–4615
Owens DM, Keyse SM (2007) Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene 26:3203–3213
Cao W, Bao C, Padalko E, Lowenstein CJ (2008) Acetylation of mitogen-activated protein kinase phosphatase-1 inhibits Toll-like receptor signaling. J Exp Med 205:1491–1503. doi:10.1084/jem.20071728
Chi H, Flavell RA (2008) Acetylation of MKP-1 and the control of inflammation. Sci Signal 1(41):e44. doi:10.1126/scisignal.141pe44
Soliman ML, Combs CK, Rosenberger TA (2013) Modulation of inflammatory cytokines and mitogen-activated protein kinases by acetate in primary astrocytes. J Neuroimmne Pharmacol 8:287–300. doi:10.1007/s11481-012-9426-4
Zhang B, West EJ, Van KC, Gurkoff GG, Zhou J, Zhang XM, Kozikowski AP, Lyeth BG (2008) HDAC inhibitor increases histone H3 acetylation and reduces microglia inflammatory response following traumatic brain injury in rats. Brain Res 1226:181–191. doi:10.1016/j.brainres.2008.05.085
Ji W, Hong L, Zhang M, Zhang W (2012) Neuroprotective effects of valproic acid following transient global ischemia in rats. Life Sci 90:463–468. doi:10.1016/j.lfs.2012.01.001
Acknowledgments
This work was supported by a grant from the KIEIKAI Research Foundation (to M. M.) and by JSPS KAKENHI Grant numbers 26450447 (to M. M.) and 15 K07768 (to Y. N.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Moriyama, M., Kurebayashi, R., Kawabe, K. et al. Acetate Attenuates Lipopolysaccharide-Induced Nitric Oxide Production Through an Anti-Oxidative Mechanism in Cultured Primary Rat Astrocytes. Neurochem Res 41, 3138–3146 (2016). https://doi.org/10.1007/s11064-016-2038-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-2038-2