Abstract
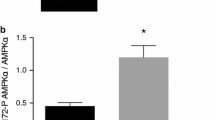
Tyrphostin 23 (T23) is a well-known inhibitor of protein tyrosine kinases. To investigate potential acute effects of T23 on the viability and the glucose metabolism of brain cells, we exposed cultured primary rat astrocytes to T23 for up to 4 h. While the viability and the morphology of the cultured astrocytes were not acutely affected by the presence of T23 in concentrations of up to 300 µM, this compound caused a rapid, time- and concentration-dependent increase in glucose consumption and lactate release. Maximal effects on glycolytic flux were found for incubations with 100 µM T23 for 2 h which doubled both glucose consumption and lactate production. The stimulation of glycolytic flux by T23 was reversible, completely abolished upon removal of the compound and not found in presence of other known inhibitors of endocytosis. Structurally related compounds such as tyrphostin 25 and catechol or modulators of AMP kinase activity did neither affect the basal nor the T23-stimulated lactate production by astrocytes. In contrast, the presence of the phosphatase inhibitor vanadate completely abolished the stimulation by T23 of astrocytic lactate production in a concentration-dependent manner. These data suggest that T23-sensitive phosphorylation/dephosphorylation events are involved in the regulation of astrocytic glycolysis.




Similar content being viewed by others
References
Levitzki A (1992) Tyrphostins: tyrosine kinase blockers as novel antiproliferative agents and dissectors of signal transduction. Fed Am Soc Exp Biol J 6:3275–3282
Banbury DN, Oakley JD, Sessions RB, Banting G (2003) Tyrphostin A23 inhibits internalization of the transferrin receptor by perturbing the interaction between tyrosine motifs and the medium chain subunit of the AP-2 adaptor complex. J Biol Chem 278:12022–12028
Jang S, Ohtani K, Fukuoh A, Mori K, Yoshizaki T, Kitamoto N, Kim Y, Suzuki Y, Wakamiya N (2014) Scavenger receptor CL-P1 mediates endocytosis by associating with AP-2 μ2. Biochim Biophys Acta 1840:3226–3237
Levitzki A (1990) Tyrphostins-potential antiproliferative agents and novel molecular tools. Biochem Pharmacol 40:913–918
Levitzki A, Gilon C (1991) Tyrphostins as molecular tools and potential antiproliferative drugs. Trends Pharmacol Sci 12:171–174
Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF (2003) Met, metastasis, motility and more. Nat Rev Mol Cell Biol 4:915–925
Young CD, Zimmerman LJ, Hoshino D, Formisano L, Hanker AB, Gatza ML, Morrison MM, Moore PD, Whitwell CA, Dave B, Stricker T, Bhola NE, Silva GO, Patel P, Brantley-Sieders DM, Levin M, Horiates M, Palma NA, Wang K, Stephens PJ, Perou CM, Weaver AM, O’Shaughnessy JA, Chang JC, Park BH, Liebler DC, Cook RS, Arteaga CL (2015) Activating PIK3CA mutations induce an EGFR/ERK paracrine signaling axis in basal-like breast cancer. Mol Cell Proteomics 14:1959–1976
Goffin JR, Zbuk K (2013) Epidermal growth factor receptor: pathway, therapies, and pipeline. Clin Ther 35:1282–1303
Cao H-H, Cheng C-Y, Su T, Fu X-Q, Guo H, Li T, Tse AK-W, Kwan H-Y, Yu H, Yu Z-L (2015) Quercetin inhibits HGF/c-Met signaling and HGF-stimulated melanoma cell migration and invasion. Mol Cancer 14:1–12
Levitzki A, Gazit A (1995) Tyrosine kinase inhibition: an approach to drug development. Science 267:1782–1788
Ligęza J, Ligęza J, Klein A (2011) Growth factor/growth factor receptor loops in autocrine growth regulation of human prostate cancer DU145 cells. Acta Biochim Pol 58:391–396
Sagara Y, Ishige K, Tsai C, Maher P (2002) Tyrphostins protect neuronal cells from oxidative stress. J Biol Chem 277:36204–36215
Pickard MR, Jenkins SI, Koller CJ, Furness DN, Chari DM (2011) Magnetic nanoparticle labeling of astrocytes derived for neural transplantation. Tissue Eng C Methods 17:89–99
Mongin AA, Reddi JM, Charniga C, Kimelberg HK (1999) [3H]taurine and d-[3H]aspartate release from astrocyte cultures are differently regulated by tyrosine kinases. Am J Physiol 276:C1226–C1230
Crépel V, Panenka W, Kelly MEM, MacVicar BA (1998) Mitogen-activated protein and tyrosine kinases in the activation of astrocyte volume-activated chloride current. J Neurosci 18:1196–1206
Zeng W-Z, Liu D-S, Duan B, Song X-L, Wang X, Wei D, Jiang W, Zhu MX, Li Y, Xu T-L (2013) Molecular mechanism of constitutive endocytosis of acid-sensing ion channel 1a and its protective function in acidosis-induced neuronal death. J Neurosci 33:7066–7078
Petters C, Dringen R (2015) Accumulation of iron oxide nanoparticles by cultured primary neurons. Neurochem Int 81:1–9
Hirrlinger J, Dringen R (2010) The cytosolic redox state of astrocytes: maintenance, regulation and functional implications for metabolite trafficking. Brain Res Rev 63:177–188
Winkler U, Hirrlinger J (2015) Crosstalk of signaling and metabolism mediated by the NAD+/NADH redox state in brain cells. Neurochem Res 40:2394–2401
Contreras L (2015) Role of AGC1/aralar in the metabolic synergies between neuron and glia. Neurochem Int 88:38–46
Dringen R, Bishop G, Koeppe M, Dang T, Robinson S (2007) The pivotal role of astrocytes in the metabolism of iron in the brain. Neurochem Res 32:1884–1890
Hohnholt MC, Dringen R (2013) Uptake and metabolism of iron and iron oxide nanoparticles in brain astrocytes. Biochem Soc Trans 41:1588–1592
Scheiber IF, Dringen R (2013) Astrocyte functions in the copper homeostasis of the brain. Neurochem Int 62:556–565
Bulcke F, Dringen R (2015) Copper oxide nanoparticles stimulate glycolytic flux and increase the cellular contents of glutathione and metallothioneins in cultured astrocytes. Neurochem Res 40:15–26
Dringen R, Pawlowski PG, Hirrlinger J (2005) Peroxide detoxification by brain cells. J Neurosci Res 79:157–165
Dringen R, Brandmann M, Hohnholt M, Blumrich E-M (2015) Glutathione-dependent detoxification processes in astrocytes. Neurochem Res 40:2570–2582
Dringen R, Spiller S, Neumann S, Koehler Y (2016) Uptake, metabolic effects and toxicity of arsenate and arsenite in astrocytes. Neurochem Res 41:465–475
Schreiner B, Romanelli E, Liberski P, Ingold-Heppner B, Sobottka-Brillout B, Hartwig T, Chandrasekar V, Johannssen H, Zeilhofer Hanns U, Aguzzi A, Heppner F, Kerschensteiner M, Becher B (2015) Astrocyte depletion impairs redox homeostasis and triggers neuronal loss in the adult CNS. Cell Rep 12:1377–1384
Allen NJ (2013) Role of glia in developmental synapse formation. Curr Opin Neurobiol 23:1027–1033
Haydon PG, Nedergaard M (2015) How do astrocytes participate in neural plasticity? Cold Spring Harb Perspect Biol 7:a020438
Verkhratsky A, Sofroniew MV, Messing A, deLanerolle NC, Rempe D, Rodriguez JJ, Nedergaard M (2012) Neurological diseases as primary gliopathies: a reassessment of neurocentrism. ASN Neuro 4
Schousboe A, Scafidi S, Bak LK, Waagepetersen HS, McKenna MC (2014) Glutamate metabolism in the brain focusing on astrocytes. Adv Neurobiol 11:13–30
Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP (2010) The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia 58:1094–1103
Halim ND, McFate T, Mohyeldin A, Okagaki P, Korotchkina LG, Patel MS, Jeoung NH, Harris RA, Schell MJ, Verma A (2010) Phosphorylation status of pyruvate dehydrogenase distinguishes metabolic phenotypes of cultured rat brain astrocytes and neurons. Glia 58:1168–1176
Carpenter KL, Jalloh I, Hutchinson PJ (2015) Glycolysis and the significance of lactate in traumatic brain injury. Front Neurosci 9:1–15
Rogatzki MJ, Ferguson BS, Goodwin ML, Gladden LB (2015) Lactate is always the end product of glycolysis. Front Neurosci 9:22
Pellerin L, Magistretti PJ (2012) Sweet sixteen for ANLS. J Cereb Blood Flow Metab 32:1152–1166
Patet C, Quintard H, Suys T, Bloch J, Daniel RT, Pellerin L, Magistretti PJ, Oddo M (2015) Neuroenergetic response to prolonged cerebral glucose depletion after severe brain injury and the role of lactate. J Neurotrauma 32:1560–1566
Glenn TC, Martin NA, McArthur DL, Hovda DA, Vespa P, Johnson ML, Horning MA, Brooks GA (2014) Endogenous nutritive support after traumatic brain injury: peripheral lactate production for glucose supply via gluconeogenesis. J Neurotrauma 32:811–819
Machler P, Wyss MT, Elsayed M, Stobart J, Gutierrez R, von Faber-Castell A, Kaelin V, Zuend M, San Martin A, Romero-Gomez I, Baeza-Lehnert F, Lengacher S, Schneider BL, Aebischer P, Magistretti PJ, Barros LF, Weber B (2016) In vivo evidence for a lactate gradient from astrocytes to neurons. Cell Metab 23:94–102
Mosienko V, Teschemacher AG, Kasparov S (2015) Is L-lactate a novel signaling molecule in the brain? J Cereb Blood Flow Metab 35:1069–1075
Gundersen V, Storm-Mathisen J, Bergersen LH (2015) Neuroglial transmission. Physiol Rev 95:695–726
Hitosugi T, Kang S, Vander Heiden MG, Chung T-W, Elf S, Lythgoe K, Dong S, Lonial S, Wang X, Chen GZ, Xie J, Gu T-L, Polakiewicz RD, Roesel JL, Boggon TJ, Khuri FR, Gilliland DG, Cantley LC, Kaufman J, Chen J (2009) Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci Signal 2:ra73
Makinoshima H, Takita M, Matsumoto S, Yagishita A, Owada S, Esumi H, Tsuchihara K (2014) Epidermal growth factor receptor (EGFR) signaling regulates global metabolic pathways in EGFR-mutated lung adenocarcinoma. J Biol Chem 289:20813–20823
Pauwels PJ, Opperdoes FR, Trouet A (1985) Effects of antimycin, glucose deprivation, and serum on cultures of neurons, astrocytes, and neuroblastoma cells. J Neurochem 44:143–148
Tadepalle N, Koehler Y, Brandmann M, Meyer N, Dringen R (2014) Arsenite stimulates glutathione export and glycolytic flux in viable primary rat brain astrocytes. Neurochem Int 76:1–11
Tulpule K, Dringen R (2012) Formate generated by cellular oxidation of formaldehyde accelerates the glycolytic flux in cultured astrocytes. Glia 60:582–593
Magistretti PJ (2009) Role of glutamate in neuron-glia metabolic coupling. Am J Clin Nutr 90:875S–880S
Brandmann M, Nehls U, Dringen R (2013) 8-Hydroxy-efavirenz, the primary metabolite of the antiretroviral drug efavirenz, stimulates the glycolytic flux in cultured rat astrocytes. Neurochem Res 38:2524–2534
Westhaus A, Blumrich EM, Dringen R (2016) The antidiabetic drug metformin stimulates glycolytic lactate production in cultured primary rat astrocytes. Neurochem Res (in press)
Almeida A, Moncada S, Bolanos JP (2004) Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat Cell Biol 6:45–51
Hamprecht B, Löffler F (1985) Primary glial cultures as a model for studying hormone action. Methods Enzymol 109:341–345
Tulpule K, Hohnholt MC, Hirrlinger J, Dringen R (2014) Primary cultures of rat astrocytes and neurons as model systems to study metabolism and metabolite export from brain cells. In: Hirrlinger J, Waagepetersen H (ed) Neuromethods 90: brain energy metabolism. Springer, New York, pp 45–72
Petters C, Dringen R (2014) Comparison of primary and secondary rat astrocyte cultures regarding glucose and glutathione metabolism and the accumulation of iron oxide nanoparticles. Neurochem Res 39:46–58
Dringen R, Kussmaul L, Hamprecht B (1998) Detoxification of exogenous hydrogen peroxide and organic hydroperoxides by cultured astroglial cells assessed by microtiter plate assay. Brain Res Brain Res Protoc 2:223–228
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Araki N, Johnson MT, Swanson JA (1996) A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol 135:1249–1260
Li HH, Li J, Wasserloos KJ, Wallace C, Sullivan MG, Bauer PM, Stolz DB, Lee JS, Watkins SC, St Croix CM, Pitt BR, Zhang LM (2013) Caveolae-dependent and -independent uptake of albumin in cultured rodent pulmonary endothelial cells. PLoS One 8:e81903
Wang LH, Rothberg KG, Anderson RG (1993) Mis-assembly of clathrin lattices onendosomes reveals a regulatory switch for coated pit formation. J Cell Biol 123:1107–1117
Shi F, Yang L, Wang J, Kouadir M, Yang Y, Fu Y, Zhou X, Yin X, Zhao D (2013) Inhibition of phagocytosis reduced the classical activation of BV2 microglia induced by amyloidogenic fragments of beta-amyloid and prion proteins. Acta Biochim Biophys Sin (Shanghai) 45:973–978
Iversen TG, Frerker N, Sandvig K (2012) Uptake of ricinB-quantum dot nanoparticles by a macropinocytosis-like mechanism. J Nanobiotechnol 10:33
Kim SH, Kim KY, Yu SN, Park SK, Choi HD, Ji JH, Ahn SC (2015) Autophagy inhibition enhances silibinin-induced apoptosis by regulating reactive oxygen species production in human prostate cancer PC-3 cells. Biochem Biophys Res Commun 468:151–156
Le PU, Guay G, Altschuler Y, Nabi IR (2002) Caveolin-1 is a negative regulator of caveolae-mediated endocytosis to the endoplasmic reticulum. J Biol Chem 277:3371–3379
Teplova VV, Tonshin AA, Grigoriev PA, Saris NE, Salkinoja-Salonen MS (2007) Bafilomycin A1 is a potassium ionophore that impairs mitochondrial functions. J Bioenerg Biomembr 39:321–329
Poole B, Ohkuma S (1981) Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J Cell Biol 90:665–669
Corton JM, Gillespie JG, Hawley SA, Hardie DG (1995) 5-Aminoimidazole-4-carboxamide ribonucleoside. Eur J Biochem 229:558–565
Liu X, Chhipa RR, Nakano I, Dasgupta B (2014) The AMPK inhibitor compound C is a potent AMPK-independent anti-glioma agent. Mol Cancer Ther 13:596–605
Vera B, Sánchez-Abarca LI, Bolaños JP, Medina JM (1996) Inhibition of astrocyte gap junctional communication by ATP depletion is reversed by calcium sequestration. FEBS Lett 392:225–228
Grapengiesser E (1998) Unmasking of a periodic Na+ entry into glucose-stimulated pancreatic β-cells after partial inhibition of the Na/K pump. Endocrinology 139:3227–3231
Johnson KJ, Peck AR, Liu C, Tran TH, Utama FE, Sjolund AB, Schaber JD, Witkiewicz AK, Rui H (2010) PTP1B suppresses prolactin activation of Stat5 in breast cancer cells. Am J Pathol 177:2971–2983
Lu L, Gao X, Zhu M, Wang S, Wu Q, Xing S, Fu X, Liu Z, Guo M (2012) Exploration of biguanido–oxovanadium complexes as potent and selective inhibitors of protein tyrosine phosphatases. Biometals 25:599–610
Ramdas L, McMurray JS, Budde RJ (1994) The degree of inhibition of protein tyrosine kinase activity by tyrphostin 23 and 25 is related to their instability. Cancer Res 54:867–869
Scheiber I, Dringen R (2011) Copper accelerates glycolytic flux in cultured astrocytes. Neurochem Res 36:894–903
Fonseca LL, Monteiro MA, Alves PM, Carrondo MJ, Santos H (2005) Cultures of rat astrocytes challenged with a steady supply of glutamate: new model to study flux distribution in the glutamate-glutamine cycle. Glia 51:286–296
Pellerin L, Magistretti PJ (1994) Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA 91:10625–10629
Brunet JF, Grollimund L, Chatton JY, Lengacher S, Magistretti PJ, Villemure JG, Pellerin L (2004) Early acquisition of typical metabolic features upon differentiation of mouse neural stem cells into astrocytes. Glia 46:8–17
Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ (2007) Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia 55:1251–1262
Ben-Yoseph O, Boxer PA, Ross BD (1996) Assessment of the role of the glutathione and pentose phosphate pathways in the protection of primary cerebrocortical cultures from oxidative stress. J Neurochem 66:2329–2337
Bouzier-Sore A-K, Bolaños JP (2015) Uncertainties in pentose-phosphate pathway flux assessment underestimate its contribution to neuronal glucose consumption: relevance for neurodegeneration and aging. Front Aging Neurosci 7:89
Dringen R, Hoepken HH, Minich T, Ruedig C (2007) Pentose phosphate pathway and NADPH metabolism. In: Lajtha A, Gibson GE, Dienel GA (ed) Handbook of neurochemistry and molecular neurobiology: brain energetics. Integration of molecular and cellular processes. Springer, US, Boston, MA, pp 41–62
Dringen R, Gebhardt R, Hamprecht B (1993) Glycogen in astrocytes: possible function as lactate supply for neighboring cells. Brain Res 623:208–214
Dringen R, Hamprecht B (1993) Differences in glycogen metabolism in astroglia-rich primary cultures and sorbitol-selected astroglial cultures derived from mouse brain. Glia 8:143–149
Bergbauer K, Dringen R, Verleysdonk S, Gebhardt R, Hamprecht B, Wiesinger H (1996) Studies on fructose metabolism in cultured astroglial cells and control hepatocytes: lack of fructokinase activity and immunoreactivity in astrocytes. Dev Neurosci 18:371–379
Wiesinger H, Hamprecht B, Dringen R (1997) Metabolic pathways for glucose in astrocytes. Glia 21:22–34
Dringen R, Bergbauer K, Wiesinger H, Hamprecht B (1994) Utilization of mannose by astroglial cells. Neurochem Res 19:23–30
Soltoff SP (2004) Evidence that tyrphostins AG10 and AG18 are mitochondrial uncouplers that alter phosphorylation-dependent cell signaling. J Biol Chem 279:10910–10918
Spindler SR, Li R, Dhahbi JM, Yamakawa A, Sauer F (2012) Novel protein kinase signaling systems regulating lifespan identified by small molecule library screening using drosophila. PLoS One 7:e29782
Qi D, Young LH (2015) AMPK: energy sensor and survival mechanism in the ischemic heart. Trends Endocrinol Metab 26:422–429
Suzuki M, Sasabe J, Miyoshi Y, Kuwasako K, Muto Y, Hamase K, Matsuoka M, Imanishi N, Aiso S (2015) Glycolytic flux controls D-serine synthesis through glyceraldehyde-3-phosphate dehydrogenase in astrocytes. Proc Natl Acad Sci USA 112:E2217–E2224
Ducommun S, Ford RJ, Bultot L, Deak M, Bertrand L, Kemp BE, Steinberg GR, Sakamoto K (2014) Enhanced activation of cellular AMPK by dual-small molecule treatment: AICAR and A769662. Am J Physiol Endocrinol Metab 306:E688–E696
Nita II, Hershfinkel M, Lewis EC, Sekler I (2015) A crosstalk between Na+ channels, Na+/K+ pump and mitochondrial Na+ transporters controls glucose-dependent cytosolic and mitochondrial Na+ signals. Cell Calcium 57:69–75
Pellerin L (2005) How astrocytes feed hungry neurons. Mol Neurobiol 32:59–72
Ruminot I, Gutierrez R, Pena-Munzenmayer G, Anazco C, Sotelo-Hitschfeld T, Lerchundi R, Niemeyer MI, Shull GE, Barros LF (2011) NBCe1 mediates the acute stimulation of astrocytic glycolysis by extracellular K+. J Neurosci 31:14264–14271
Bolaños JP, Almeida A (2006) Modulation of astroglial energy metabolism by nitric oxide. Antioxid Redox Signal 8:955–965
Rieske JS, Zaugg WS (1962) The inhibition by antimycin A of the cleavage of one of the complexes of the respiratory chain. Biochem Biophys Res Commun 8:421–426
Potter VR, Reif AE (1952) Inhibition of an electron transport component by antimycin A. J Biol Chem 194:287–297
De Rosa V, Iommelli F, Monti M, Fonti R, Votta G, Stoppelli MP, Del Vecchio S (2015) Reversal of Warburg effect and reactivation of oxidative phosphorylation by differential inhibition of EGFR signaling pathways in non-small cell lung cancer. Clin Cancer Res 21:5110–5120
Korotchkina LG, Patel MS (2001) Site specificity of four pyruvate dehydrogenase kinase isoenzymes toward the three phosphorylation sites of human pyruvate dehydrogenase. J Biol Chem 276:37223–37229
Nucifora PG, Fox AP (1999) Tyrosine phosphorylation regulates rapid endocytosis in adrenal chromaffin cells. J Neurosci 19:9739–9746
Skogseth H, Holt RU, Larsson E, Halgunset J (2006) Tyrosine kinase inhibitors alter adhesivity of prostatic cancer cells to extracellular matrix components. APMIS 114:225–233
Acknowledgments
The authors would like to thank Yvonne Koehler (University of Bremen) for her support in establishing the assay to determine intracellular glucose and Prof. Dr. Soerge Kelm (University of Bremen) for providing access to his fluorescence microtiter plate reader.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Blumrich, EM., Kadam, R. & Dringen, R. The Protein Tyrosine Kinase Inhibitor Tyrphostin 23 Strongly Accelerates Glycolytic Lactate Production in Cultured Primary Astrocytes. Neurochem Res 41, 2607–2618 (2016). https://doi.org/10.1007/s11064-016-1972-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1972-3