Abstract
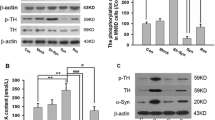
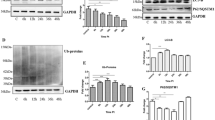
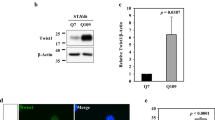
Cellular prion protein (PrPC) is a glycoprotein of the plasma membrane that plays pleiotropic functions by interacting with multiple signaling complexes at the cell surface. Recently, a number of studies have reported the involvement of PrPC in dopamine metabolism and signaling, including its interactions with tyrosine hydroxylase (TH) and dopamine receptors. However, the outcomes reported by independent studies are still debatable. Therefore in this study, we investigated the effects of PrPC on the TH expression during the differentiation of N2a cells with dibutyryl-cAMP, a well-known cAMP analog that activates TH transcription. Upon differentiation, TH was induced with concomitant reduction of PrPC at protein level, but not at mRNA level. shRNA-mediated PrPC reduction increased the basal level of TH at both mRNA and protein levels without dibutyryl-cAMP treatment. This phenotype was reversed by re-expression of PrPC. PrPC knockdown also potentiated the effect of dibutyryl-cAMP on TH expression. Our findings suggest that PrPC has suppressive effects on TH expression. As a consequence, altered PrPC functions may affect the regulation of dopamine metabolism and related neurological disorders.




Similar content being viewed by others
References
Prusiner SB (1998) Prions. Proc Natl Acad Sci USA 95(23):13363–13383. doi:10.1073/pnas.95.23.13363
Romano SA, Cordeiro Y, Lima LMTR et al (2009) Reciprocal remodeling upon binding of the prion protein to its signaling partner hop/STI1. FASEB J 23:4308–4316. doi:10.1096/fj.09-138974
Taubner LM, Bienkiewicz EA, Copié V, Caughey B (2010) Structure of the flexible amino-terminal domain of prion protein bound to a sulfated glycan. J Mol Biol 395:475–490. doi:10.1016/j.jmb.2009.10.075
Thakur AK, Srivastava AK, Srinivas V et al (2011) Copper alters aggregation behavior of prion protein and induces novel interactions between Its N- and C-terminal regions. J Biol Chem 286:38533–38545. doi:10.1074/jbc.M111.265645
da Luz MHM, Peres IT, Santos TG et al (2015) Dopamine induces the accumulation of insoluble prion protein and affects autophagic flux. Front Cell Neurosci 9:12. doi:10.3389/fncel.2015.00012
Gunapala KM, Chang D, Hsu CT et al (2010) Striatal pathology underlies prion infection-mediated hyperactivity in mice. Prion 4:302–315
Durand-Gorde JM, Bert J, Nieoullon A (1985) Changes in tyrosine hydroxylase, glutamic acid decarboxylase and choline acetyltransferase after local microinoculation of scrapie agent into the nigrostriatal system of the golden hamster. Brain Res 341:243–251
Vicario M, Zagari A, Granata V et al (2014) A novel prion protein-tyrosine hydroxylase interaction. CNS Neurol Disord Drug Targets 13:896–908
Beckman D, Santos LE, Americo TA et al (2015) Prion protein modulates monoaminergic systems and depressive-like behavior in mice. J Biol Chem 290:20488–20498. doi:10.1074/jbc.M115.666156
Rial D, Pamplona FA, Moreira ELG et al (2014) Cellular prion protein is present in dopaminergic neurons and modulates the dopaminergic system. Eur J Neurosci 40:2479–2486. doi:10.1111/ejn.12600
Nuvolone M, Aguzzi A (2015) Altered monoaminergic systems and depressive-like behavior in congenic prion protein knock-out mice. J Biol Chem 290:26350. doi:10.1074/jbc.L115.689117
Beckman D, Santos LE, Americo TA et al (2015) Reply to altered monoaminergic systems and depressive-like behavior in congenic prion protein knock-out mice. J Biol Chem 290:26351. doi:10.1074/jbc.L115.689232
Caughey B (1991) In vitro expression and biosynthesis of prion protein. Curr Top Microbiol Immunol 172:93–107
Caughey B, Race RE, Ernst D et al (1989) Prion protein biosynthesis in scrapie-infected and uninfected neuroblastoma cells. J Virol 63:175–181
Tremblay RG, Sikorska M, Sandhu JK et al (2010) Differentiation of mouse Neuro 2A cells into dopamine neurons. J Neurosci Methods 186:60–67. doi:10.1016/j.jneumeth.2009.11.004
Zager A, Andersen ML, Lima MMS et al (2009) Modulation of sickness behavior by sleep: the role of neurochemical and neuroinflammatory pathways in mice. Eur Neuropsychopharmacol 19:589–602. doi:10.1016/j.euroneuro.2009.03.005
Sunyach C, Jen A, Deng J et al (2003) The mechanism of internalization of glycosylphosphatidylinositol-anchored prion protein. EMBO J 22:3591–3601. doi:10.1093/emboj/cdg344
Atabay C, Cagnoli CM, Kharlamov E et al (1996) Removal of serum from primary cultures of cerebellar granule neurons induces oxidative stress and DNA fragmentation: protection with antioxidants and glutamate receptor antagonists. J Neurosci Res 43:465–475. doi:10.1002/(SICI)1097-4547(19960215)43:4<465:AID-JNR7>3.0.CO;2-D
Lebel M, Gauthier Y, Moreau A, Drouin J (2001) Pitx3 activates mouse tyrosine hydroxylase promoter via a high-affinity binding site. J Neurochem 77:558–567
Cabral ALB, Lee KS, Martins VR (2002) Regulation of the cellular prion protein gene expression depends on chromatin conformation. J Biol Chem 277:5675–5682. doi:10.1074/jbc.M104815200
Moran EC, Kamiguti AS, Cawley JC, Pettitt AR (2002) Cytoprotective antioxidant activity of serum albumin and autocrine catalase in chronic lymphocytic leukaemia. Br J Haematol 116:316–328
Halliwell B, Gutteridge JM (1990) The antioxidants of human extracellular fluids. Arch Biochem Biophys 280:1–8
Evangelopoulos ME, Weis J, Krüttgen A (2005) Signalling pathways leading to neuroblastoma differentiation after serum withdrawal: HDL blocks neuroblastoma differentiation by inhibition of EGFR. Oncogene 24:3309–3318. doi:10.1038/sj.onc.1208494
Crespi A, Ferrari I, Lonati P et al (2012) LIN7 regulates the filopodium- and neurite-promoting activity of IRSp53. J Cell Sci 125:4543–4554. doi:10.1242/jcs.106484
Mahal SP, Baker CA, Demczyk CA et al (2007) Prion strain discrimination in cell culture: the cell panel assay. Proc Natl Acad Sci 104:20908–20913. doi:10.1073/pnas.0710054104
Denman R, Potempska A, Wolfe G et al (1991) Distribution and activity of alternatively spliced Alzheimer amyloid peptide precursor and scrapie PrP mRNAs on rat brain polysomes. Arch Biochem Biophys 288:29–38
Ford MJ, Burton LJ, Li H et al (2002) A marked disparity between the expression of prion protein and its message by neurones of the CNS. Neuroscience 111:533–551
Ma J, Lindquist S (1999) De novo generation of a PrPSc-like conformation in living cells. Nat Cell Biol 1:358–361. doi:10.1038/14053
Rudd PM, Endo T, Colominas C et al (1999) Glycosylation differences between the normal and pathogenic prion protein isoforms. Proc Natl Acad Sci USA 96:13044–13049
Rudd PM, Merry AH, Wormald MR, Dwek RA (2002) Glycosylation and prion protein. Curr Opin Struct Biol 12:578–586
Capellari S, Zaidi SI, Urig CB et al (1999) Prion protein glycosylation is sensitive to redox change. J Biol Chem 274:34846–34850
Young JE, Martinez RA, La Spada AR (2009) Nutrient deprivation induces neuronal autophagy and implicates reduced insulin signaling in neuroprotective autophagy activation. J Biol Chem 284:2363–2373. doi:10.1074/jbc.M806088200
Huber B, Volz A-C, Kluger PJ (2015) How do culture media influence in vitro perivascular cell behavior? Cell Biol Int 39:1395–1407. doi:10.1002/cbin.10515
Costa AR, Withers J, Rodrigues ME et al (2013) The impact of cell adaptation to serum-free conditions on the glycosylation profile of a monoclonal antibody produced by Chinese hamster ovary cells. New Biotechnol 30:563–572. doi:10.1016/j.nbt.2012.12.002
Linden R, Martins VR, Prado MAM et al (2008) Physiology of the prion protein. Physiol Rev 88:673–728. doi:10.1152/physrev.00007.2007
Linden R, Cordeiro Y, Lima LMTR (2012) Allosteric function and dysfunction of the prion protein. Cell Mol Life Sci 69:1105–1124. doi:10.1007/s00018-011-0847-7
Bamber BA, Masters BA, Hoyle GW et al (1994) Leukemia inhibitory factor induces neurotransmitter switching in transgenic mice. Proc Natl Acad Sci USA 91:7839–7843
Klinghoffer RA, Magnus J, Schelter J et al (2010) Reduced seed region-based off-target activity with lentivirus-mediated RNAi. RNA 16:879–884. doi:10.1261/rna.1977810
Schnell PO, Ignacak ML, Bauer AL et al (2003) Regulation of tyrosine hydroxylase promoter activity by the von Hippel–Lindau tumor suppressor protein and hypoxia-inducible transcription factors. J Neurochem 85:483–491
Seo J-S, Seol J-W, Moon M-H et al (2010) Hypoxia protects neuronal cells from human prion protein fragment-induced apoptosis. J Neurochem 112:715–722. doi:10.1111/j.1471-4159.2009.06496.x
Jeong J-K, Park S-Y (2012) Transcriptional regulation of specific protein 1 (SP1) by hypoxia-inducible factor 1 alpha (HIF-1α) leads to PRNP expression and neuroprotection from toxic prion peptide. Biochem Biophys Res Commun 429:93–98. doi:10.1016/j.bbrc.2012.10.086
Jeong J-K, Seo J-S, Moon M-H et al (2012) Hypoxia-inducible factor-1 α regulates prion protein expression to protect against neuron cell damage. Neurobiol Aging 33(1006):e1–e10. doi:10.1016/j.neurobiolaging.2011.09.037
Horak P, Crawford AR, Vadysirisack DD et al (2010) Negative feedback control of HIF-1 through REDD1-regulated ROS suppresses tumorigenesis. Proc Natl Acad Sci USA 107:4675–4680. doi:10.1073/pnas.0907705107
Wang H, Flach H, Onizawa M et al (2014) Negative regulation of Hif1a expression and TH17 differentiation by the hypoxia-regulated microRNA miR-210. Nat Immunol 15:393–401. doi:10.1038/ni.2846
Sasaki K, Doh-ura K, Furuta A et al (2003) Neuropathological features of a case with schizophrenia and prion protein gene P102L mutation before onset of Gerstmann–Sträussler–Scheinker disease. Acta Neuropathol 106:92–96. doi:10.1007/s00401-003-0697-y
Iida T, Doh-ura K, Kawashima T et al (2001) An atypical case of sporadic Creutzfeldt–Jakob disease with Parkinson’s disease. Neuropathology 21:294–297
Acknowledgments
This study was supported by Grants from FAPESP [Fundação de Amparo à Pesquisa do Estado de São Paulo: 2013/22413-5 (K.S.L); 2013/07937-8 (I.G)], CAPES (Coordenação de Aperfeiçoamento de pessoal de Nível Superior), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico: 467566/2014-3) and EMU (programa de equipamentos multiusuários). We thank Dr. Ricardo Borges Machado from UNIFESP, who carried out the quantification of dopamine and its metabolites.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflict of interests to disclose.
Rights and permissions
About this article
Cite this article
da Luz, M.H.M., Glezer, I., Xavier, A.M. et al. Expression of Tyrosine Hydroxylase is Negatively Regulated Via Prion Protein. Neurochem Res 41, 1691–1699 (2016). https://doi.org/10.1007/s11064-016-1885-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1885-1