Abstract
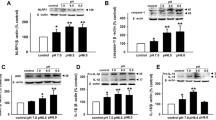
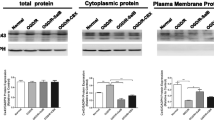
The destruction of calcium homeostasis is an important factor leading to neurological diseases. Store-operated Ca2+ (SOC) channels are essential for Ca2+ homeostasis in many cell types. However, whether SOC channels are involved in astrocyte activation induced by lipopolysaccharide (LPS) still remains unknown. In this study, we used LPS as an exogenous stimulation to investigate the role of SOC channels in astrocyte activation. Using calcium imaging technology, we first found that SOC channels blockers, 1-[h-[3-(4-methoxyphenyl)propoxy]-4-methoxyphenethyl]-1H-imidazole (SKF-96365) and 2-aminoethyldiphenyl borate (2-APB), inhibited LPS induced [Ca2+]i increase, which prompted us to speculate that SOC channels may be involved in LPS induced astrocyte activation. Further experiments confirmed our speculation shown as SOC channels blockers inhibited LPS induced astrocyte activation characterized as cell proliferation by MTS and BrdU assay, raise in glial fibrillary acidic protein expression by immunofluorescence and Western Blot and secretion of interleukin 6 (IL-6) and interleukin 1β (IL-1β) by ELISA. So, our studies showed that SOC channels are involved in LPS-induced astrocyte activation.




Similar content being viewed by others
References
Yu AC, Lee YL, Eng LF (1993) Astrogliosis in culture: I. The model and the effect of antisense oligonucleotides on glial fibrillary acidic protein synthesis. J Neurosci Res 34(3):295–303. doi:10.1002/jnr.490340306
Suryadevara R, Holter S, Borgmann K, Persidsky R, Labenz-Zink C, Persidsky Y, Gendelman HE, Wu L, Ghorpade A (2003) Regulation of tissue inhibitor of metalloproteinase-1 by astrocytes: links to HIV-1 dementia. Glia 44(1):47–56. doi:10.1002/glia.10266
Neil SJ, Aasa-Chapman MM, Clapham PR, Nibbs RJ, McKnight A, Weiss RA (2005) The promiscuous CC chemokine receptor D6 is a functional coreceptor for primary isolates of human immunodeficiency virus type 1 (HIV-1) and HIV-2 on astrocytes. J Virol 79(15):9618–9624. doi:10.1128/JVI.79.15.9618-9624.2005
Eng LF, Ghirnikar RS (1994) GFAP and astrogliosis. Brain Pathol 4(3):229–237
Villereal ML, Byron KL (1992) Calcium signals in growth factor signal transduction. Rev Physiol Biochem Pharmacol 119:67–121
Berridge MJ (1995) Calcium signalling and cell proliferation. BioEssays 17(6):491–500. doi:10.1002/bies.950170605
Berridge MJ (2001) The versatility and complexity of calcium signalling. Novartis Found Symp 239:52–64; discussion 64–57, 150–159
Berridge MJ, Lipp P, Bootman MD (2000) The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1(1):11–21. doi:10.1038/35036035
Putney JW Jr (2004) The enigmatic TRPCs: multifunctional cation channels. Trends Cell Biol 14(6):282–286. doi:10.1016/j.tcb.2004.04.002
Parekh AB (2003) Store-operated Ca2+ entry: dynamic interplay between endoplasmic reticulum, mitochondria and plasma membrane. J Physiol 547(Pt 2):333–348. doi:10.1113/jphysiol.2002.034140
Parekh AB, Penner R (1997) Store depletion and calcium influx. Physiol Rev 77(4):901–930
Kozuka N, Kudo Y, Morita M (2007) Multiple inhibitory pathways for lipopolysaccharide- and pro-inflammatory cytokine-induced nitric oxide production in cultured astrocytes. Neuroscience 144(3):911–919. doi:10.1016/j.neuroscience.2006.10.040
Hosoi T, Suzuki S, Okuma Y, Kawakami A, Ogawa N, Ozawa K, Nomura Y (2005) LPS induces stefin A3 expression in mouse primary cultured glial cells. Brain Res Mol Brain Res 140(1–2):138–141. doi:10.1016/j.molbrainres.2005.07.007
Zhao ST, Zhao L, Li JH (2013) Neuroprotective Peptide humanin inhibits inflammatory response in astrocytes induced by lipopolysaccharide. Neurochem Res 38(3):581–588. doi:10.1007/s11064-012-0951-6
Zhao ST, Huang XT, Zhang C, Ke Y (2012) Humanin protects cortical neurons from ischemia and reperfusion injury by the increased activity of superoxide dismutase. Neurochem Res 37(1):153–160. doi:10.1007/s11064-011-0593-0
Zhao ST, Chen M, Li SJ, Zhang MH, Li BX, Das M, Bean JC, Kong JM, Zhu XH, Gao TM (2009) Mitochondrial BNIP3 upregulation precedes endonuclease G translocation in hippocampal neuronal death following oxygen-glucose deprivation. BMC Neurosci 10:113. doi:10.1186/1471-2202-10-113
Eng LF, Yu AC, Lee YL (1992) Astrocytic response to injury. Prog Brain Res 94:353–365
Dong Y, Benveniste EN (2001) Immune function of astrocytes. Glia 36(2):180–190. doi:10.1002/glia.1107
Koizumi S, Fujishita K (2007) Gliotransmitter ATP-mediated cell-to-cell communication. Brain Nerve 59(7):707–715
Koehler RC, Roman RJ, Harder DR (2009) Astrocytes and the regulation of cerebral blood flow. Trends Neurosci 32(3):160–169. doi:10.1016/j.tins.2008.11.005
Rolls A, Shechter R, Schwartz M (2009) The bright side of the glial scar in CNS repair. Nat Rev Neurosci 10(3):235–241. doi:10.1038/nrn2591
Buffo A, Rolando C, Ceruti S (2010) Astrocytes in the damaged brain: molecular and cellular insights into their reactive response and healing potential. Biochem Pharmacol 79(2):77–89. doi:10.1016/j.bcp.2009.09.014
Bading H, Ginty DD, Greenberg ME (1993) Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science 260(5105):181–186
Bezprozvanny I (2009) Calcium signaling and neurodegenerative diseases. Trends Mol Med 15(3):89–100. doi:10.1016/j.molmed.2009.01.001
Marambaud P, Dreses-Werringloer U, Vingtdeux V (2009) Calcium signaling in neurodegeneration. Mol Neurodegener 4:20. doi:10.1186/1750-1326-4-20
Parekh AB, Putney JW Jr (2005) Store-operated calcium channels. Physiol Rev 85(2):757–810. doi:10.1152/physrev.00057.2003
Berridge MJ (2005) Unlocking the secrets of cell signaling. Annu Rev Physiol 67:1–21. doi:10.1146/annurev.physiol.67.040103.152647
Strokin M, Sergeeva M, Reiser G (2011) Proinflammatory treatment of astrocytes with lipopolysaccharide results in augmented Ca2+ signaling through increased expression of via phospholipase A2 (iPLA2). Am J Physiol Cell Physiol 300(3):C542–C549. doi:10.1152/ajpcell.00428.2010
Sherwin C, Fern R (2005) Acute lipopolysaccharide-mediated injury in neonatal white matter glia: role of TNF-alpha, IL-1beta, and calcium. J Immunol 175(1):155–161
Putney JW Jr, McKay RR (1999) Capacitative calcium entry channels. BioEssays 21(1):38–46. doi:10.1002/(SICI)1521-1878(199901)21:1<38:AID-BIES5>3.0.CO;2-S
Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K (1997) 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem 122(3):498–505
Gregory RB, Rychkov G, Barritt GJ (2001) Evidence that 2-aminoethyl diphenylborate is a novel inhibitor of store-operated Ca2+ channels in liver cells, and acts through a mechanism which does not involve inositol trisphosphate receptors. Biochem J 354(Pt 2):285–290
Braun FJ, Broad LM, Armstrong DL, Putney JW Jr (2001) Stable activation of single Ca2+ release-activated Ca2+ channels in divalent cation-free solutions. J Biol Chem 276(2):1063–1070. doi:10.1074/jbc.M008348200
Broad LM, Braun FJ, Lievremont JP, Bird GS, Kurosaki T, Putney JW Jr (2001) Role of the phospholipase C-inositol 1,4,5-trisphosphate pathway in calcium release-activated calcium current and capacitative calcium entry. J Biol Chem 276(19):15945–15952. doi:10.1074/jbc.M011571200
Ma HT, Patterson RL, van Rossum DB, Birnbaumer L, Mikoshiba K, Gill DL (2000) Requirement of the inositol trisphosphate receptor for activation of store-operated Ca2+ channels. Science 287(5458):1647–1651. doi:10.1126/science.287.5458.1647
Prakriya M, Lewis RS (2001) Potentiation and inhibition of Ca(2+) release-activated Ca(2+) channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP(3) receptors. J Physiol 536(Pt 1):3–19. doi:10.1111/j.1469-7793.2001.t01-1-00003.x
Bakowski D, Glitsch MD, Parekh AB (2001) An examination of the secretion-like coupling model for the activation of the Ca2+ release-activated Ca2+ current I(CRAC) in RBL-1 cells. J Physiol 532(Pt 1):55–71. doi:10.1111/j.1469-7793.2001.0055g.x
Ma HT, Venkatachalam K, Li HS, Montell C, Kurosaki T, Patterson RL, Gill DL (2001) Assessment of the role of the inositol 1,4,5-trisphosphate receptor in the activation of transient receptor potential channels and store-operated Ca2+ entry channels. J Biol Chem 276(22):18888–18896. doi:10.1074/jbc.M100944200
Voets T, Prenen J, Fleig A, Vennekens R, Watanabe H, Hoenderop JG, Bindels RJ, Droogmans G, Penner R, Nilius B (2001) CaT1 and the calcium release-activated calcium channel manifest distinct pore properties. J Biol Chem 276(51):47767–47770. doi:10.1074/jbc.C100607200
Missiaen L, Callewaert G, De Smedt H, Parys JB (2001) 2-Aminoethoxydiphenyl borate affects the inositol 1,4,5-trisphosphate receptor, the intracellular Ca2+ pump and the non-specific Ca2+ leak from the non-mitochondrial Ca2+ stores in permeabilized A7r5 cells. Cell Calcium 29(2):111–116. doi:10.1054/ceca.2000.0163
Hermosura MC, Monteilh-Zoller MK, Scharenberg AM, Penner R, Fleig A (2002) Dissociation of the store-operated calcium current I(CRAC) and the Mg-nucleotide-regulated metal ion current MagNuM. J Physiol 539(Pt 2):445–458. doi:10.1113/jphysiol.2001.013361
Schindl R, Kahr H, Graz I, Groschner K, Romanin C (2002) Store depletion-activated CaT1 currents in rat basophilic leukemia mast cells are inhibited by 2-aminoethoxydiphenyl borate. Evidence for a regulatory component that controls activation of both CaT1 and CRAC (Ca(2+) release-activated Ca(2+) channel) channels. J Biol Chem 277(30):26950–26958. doi:10.1074/jbc.M203700200
Dobrydneva Y, Blackmore P (2001) 2-Aminoethoxydiphenyl borate directly inhibits store-operated calcium entry channels in human platelets. Mol Pharmacol 60(3):541–552
Iwasaki H, Mori Y, Hara Y, Uchida K, Zhou H, Mikoshiba K (2001) 2-Aminoethoxydiphenyl borate (2-APB) inhibits capacitative calcium entry independently of the function of inositol 1,4,5-trisphosphate receptors. Recept Channels 7(6):429–439
DeHaven WI, Smyth JT, Boyles RR, Bird GS, Putney JW Jr (2008) Complex actions of 2-aminoethyldiphenyl borate on store-operated calcium entry. J Biol Chem 283(28):19265–19273. doi:10.1074/jbc.M801535200
Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM (2002) 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J 16(10):1145–1150. doi:10.1096/fj.02-0037rev
Peinelt C, Lis A, Beck A, Fleig A, Penner R (2008) 2-Aminoethoxydiphenyl borate directly facilitates and indirectly inhibits STIM1-dependent gating of CRAC channels. J Physiol 586(13):3061–3073. doi:10.1113/jphysiol.2008.151365
Wang JP (2003) Characterization of maleimide-activated Ca2+ entry in neutrophils. Biochem Pharmacol 65(12):1923–1929. doi:10.1016/S0006-2952(03)00194-1
Kukkonen JP, Lund PE, Akerman KE (2001) 2-aminoethoxydiphenyl borate reveals heterogeneity in receptor-activated Ca(2+) discharge and store-operated Ca(2+) influx. Cell Calcium 30(2):117–129. doi:10.1054/ceca.2001.0219
Baba A, Yasui T, Fujisawa S, Yamada RX, Yamada MK, Nishiyama N, Matsuki N, Ikegaya Y (2003) Activity-evoked capacitative Ca2+ entry: implications in synaptic plasticity. J Neurosci 23(21):7737–7741
Eng LF, Ghirnikar RS, Lee YL (2000) Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochem Res 25(9–10):1439–1451
Liedtke W, Edelmann W, Bieri PL, Chiu FC, Cowan NJ, Kucherlapati R, Raine CS (1996) GFAP is necessary for the integrity of CNS white matter architecture and long-term maintenance of myelination. Neuron 17(4):607–615. doi:10.1016/S0896-6273(00)80194-4
Messing A, Goldman JE, Johnson AB, Brenner M (2001) Alexander disease: new insights from genetics. J Neuropathol Exp Neurol 60(6):563–573
Sriram K, Benkovic SA, Hebert MA, Miller DB, O’Callaghan JP (2004) Induction of gp130-related cytokines and activation of JAK2/STAT3 pathway in astrocytes precedes up-regulation of glial fibrillary acidic protein in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of neurodegeneration: key signaling pathway for astrogliosis in vivo? J Biol Chem 279(19):19936–19947. doi:10.1074/jbc.M309304200
Van Wagoner NJ, Benveniste EN (1999) Interleukin-6 expression and regulation in astrocytes. J Neuroimmunol 100(1–2):124–139. doi:10.1016/S0165-5728(99)00187-3
Rothwell NJ, Luheshi GN (2000) Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci 23(12):618–625. doi:10.1016/S0166-2236(00)01661-1
Bergsbaken T, Fink SL, Cookson BT (2009) Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 7(2):99–109. doi:10.1038/nrmicro2070
Blum-Degen D, Muller T, Kuhn W, Gerlach M, Przuntek H, Riederer P (1995) Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci Lett 202(1–2):17–20
Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM (2001) The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J 15(1):43–58. doi:10.1096/fj.99-1003rev
Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T (2000) Inflammation and Alzheimer’s disease. Neurobiol Aging 21(3):383–421
Sattayaprasert P, Choi HB, Chongthammakun S, McLarnon JG (2005) Platelet-activating factor enhancement of calcium influx and interleukin-6 expression, but not production, in human microglia. J Neuroinflammation 2(1):11. doi:10.1186/1742-2094-2-11
Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, Gill DL, Ambudkar IS (2007) Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J Biol Chem 282(12):9105–9116. doi:10.1074/jbc.M608942200
Pizzo P, Burgo A, Pozzan T, Fasolato C (2001) Role of capacitative calcium entry on glutamate-induced calcium influx in type-I rat cortical astrocytes. J Neurochem 79(1):98–109
Barajas M, Andrade A, Hernandez–Hernandez O, Felix R, Arias-Montano JA (2008) Histamine-induced Ca2+ entry in human astrocytoma U373 MG cells: evidence for involvement of store-operated channels. J Neurosci Res 86(15):3456–3468. doi:10.1002/jnr.21784
Grimaldi M, Maratos M, Verma A (2003) Transient receptor potential channel activation causes a novel form of [Ca2+]I oscillations and is not involved in capacitative Ca2+ entry in glial cells. J Neurosci 23(11):4737–4745
Golovina VA (2005) Visualization of localized store-operated calcium entry in mouse astrocytes. Close proximity to the endoplasmic reticulum. J Physiol 564(Pt 3):737–749. doi:10.1113/jphysiol.2005.085035
Ambudkar IS (2006) Ca2+ signaling microdomains:platforms for the assembly and regulation of TRPC channels. Trends Pharmacol Sci 27(1):25–32. doi:10.1016/j.tips.2005.11.008
Song X, Zhao Y, Narcisse L, Duffy H, Kress Y, Lee S, Brosnan CF (2005) Canonical transient receptor potential channel 4 (TRPC4) co-localizes with the scaffolding protein ZO-1 in human fetal astrocytes in culture. Glia 49(3):418–429. doi:10.1002/glia.20128
Beskina O, Miller A, Mazzocco-Spezzia A, Pulina MV, Golovina VA (2007) Mechanisms of interleukin-1beta-induced Ca2+ signals in mouse cortical astrocytes: roles of store- and receptor-operated Ca2+ entry. Am J Physiol Cell Physiol 293(3):C1103–C1111. doi:10.1152/ajpcell.00249.2007
Malarkey EB, Ni Y, Parpura V (2008) Ca2+ entry through TRPC1 channels contributes to intracellular Ca2+ dynamics and consequent glutamate release from rat astrocytes. Glia 56(8):821–835. doi:10.1002/glia.20656
Moreno C, Sampieri A, Vivas O, Pena-Segura C, Vaca L (2012) STIM1 and Orai1 mediate thrombin-induced Ca(2+) influx in rat cortical astrocytes. Cell Calcium 52(6):457–467. doi:10.1016/j.ceca.2012.08.004
Nakao K, Shirakawa H, Sugishita A, Matsutani I, Niidome T, Nakagawa T, Kaneko S (2008) Ca2+ mobilization mediated by transient receptor potential canonical 3 is associated with thrombin-induced morphological changes in 1321N1 human astrocytoma cells. J Neurosci Res 86(12):2722–2732. doi:10.1002/jnr.21711
Rychkov G, Barritt GJ (2007) TRPC1 Ca(2+)-permeable channels in animal cells. Handb Exp Pharmacol 179:23–52. doi:10.1007/978-3-540-34891-7_2
Xu SZ, Zeng F, Boulay G, Grimm C, Harteneck C, Beech DJ (2005) Block of TRPC5 channels by 2-aminoethoxydiphenyl borate: a differential, extracellular and voltage-dependent effect. Br J Pharmacol 145(4):405–414. doi:10.1038/sj.bjp.0706197
Merritt JE, Armstrong WP, Benham CD, Hallam TJ, Jacob R, Jaxa-Chamiec A, Leigh BK, McCarthy SA, Moores KE, Rink TJ (1990) SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem J 271(2):515–522
Acknowledgments
We would like to thank Karrune Woan and Jun-Min Zhou for assistance in preparation of the Main manuscript. This work was supported by Natural Science Foundation of Guangdong Province (NO. 8151018201000039), Key Research Foundation of Guangzhou Education Bureau (2012C043) to JHL, National Natural Science Foundation of China (NO. 81100986), Research Foundation of Guangzhou Education Bureau (NO. 10A154) and Breeding Project of Guangdong Province (NO. LYM11105) to ZST.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jian-Hua Li and Shen-Ting Zhao contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11064_2013_1130_MOESM1_ESM.ppt
Supplemental Fig 1 Increased cell viability by LPS for 48 h. The viability was examined by MTS assay. n=3. Results are mean±SE of different independent experiments. *P < 0.05 vs. control group (PPT 64 kb)
11064_2013_1130_MOESM2_ESM.ppt
Supplemental Fig 2 Increased percentage of BrdU positive cells by LPS for 48 h. Data of BrdU positive cells were presented as percentage of the control group. n=15. Results are mean±SE of different independent experiments. *P < 0.05 vs. control group (PPT 64 kb)
11064_2013_1130_MOESM3_ESM.ppt
Supplemental Fig 3 GFAP increased expression induced by LPS. Cells were incubated with 0.5μg/ml LPS for different incubation time. (a) The immunofluorescence of GFAP (green), Co-staining with DAPI (blue) allowed the identification of the nucleus. (b) Representative western blot showing the expression of GFAP. (c) Quantification of the expression of GFAP. Results are mean±SE of four different independent experiments. *P < 0.05 vs. 0 h group (PPT 6914 kb)
11064_2013_1130_MOESM4_ESM.ppt
Supplemental Fig 4 Increased IL-6 and IL-1β by LPS. Cells were incubated with 0.5μg/ml LPS for different incubation time. (a) The level of IL-6. n=4. (b) The level of IL-1β. n=4. Results are mean±SE of four different independent experiments. *P < 0.05 vs. 0 h group (PPT 274 kb)
Rights and permissions
About this article
Cite this article
Li, JH., Zhao, ST., Wu, CY. et al. Store-Operated Ca2+ Channels Blockers Inhibit Lipopolysaccharide Induced Astrocyte Activation. Neurochem Res 38, 2216–2226 (2013). https://doi.org/10.1007/s11064-013-1130-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-013-1130-0