Abstract
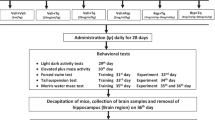
The aim of this study was to evaluate the neuroprotective effects of nerolidol in mice hippocampus against oxidative stress in neuronal cells compared to ascorbic acid (positive control) as well as evaluated the nerolidol sedative effects by open field test compared to diazepam (positive control). Thirty minutes prior to behavioral observation on open field test, mice were intraperitoneally treated with vehicle, nerolidol (25, 50 and 75 mg/kg), diazepam (1 mg/kg) or ascorbic acid (250 mg/kg). To clarify the action mechanism of of nerolidol on oxidative stress in animals subjected to the open field test, Western blot analysis of Mn-superoxide dismutase and catalase in mice hippocampus were performed. In nerolidol group, there was a significant decrease in lipid peroxidation and nitrite levels when compared to negative control (vehicle). However, a significant increase was observed in superoxide dismutase and catalase activities in this group when compared to the other groups. Vehicle, diazepam, ascorbic acid and nerolidol groups did not affected Mn-superoxide dismutase, catalase mRNA or protein levels. Our findings strongly support the hypothesis that oxidative stress occurs in hippocampus. Nerolidol showed sedative effects in animals subjected to the open field test. Oxidative process plays a crucial role on neuronal pathological consequence, and implies that antioxidant effects could be achieved using this sesquiterpene.






Similar content being viewed by others
References
Freitas RM, Souza FCF, Vasconcelos SMM, Viana GSB, Fonteles MMF (2005) Oxidative stress in the hippocampus after status epilepticus in rats. FEBS J 272:1307–1312
Surmeier DJ, Guzman JN, Sanchez-Padilla J, Schumacker PT (2011) The role of calcium and mitochondrial oxidant stress in the loss of substantia nigra pars compacta dopaminergic neurons in Parkinson’s disease. Neuroscience 198:221–231
Avery SV (2011) Molecular targets of oxidative stress. Biochem J 434:201–210
Militão GCG, Ferreira PMP, Freitas RM (2010) Effects of lipoic acid on oxidative stress in rat striatum after pilocarpine-induced seizures. Neurochem Int 56:16–20
Ferreira PMP, Santos AG, Tininis AG, Costa PM, Cavalheiro AJ, Bolzani VS, Moraes MO, Costa-Lotufo LV, Montenegro RC, Pessoa C (2010) Casearin X exhibits cytotoxic effects in leukemia cells triggered by apoptosis. Chem Biol Interact 188:497–504
Ferreira PMP, Costa-Lotufo LV, Moraes MO, Barros FW, Martins AM, Cavalheiro AJ, Bolzani VS, Santos AG, Pessoa C (2011) Folk uses and pharmacological properties of Casearia sylvestris: a medicinal review. An Acad Bras Cienc 83:1373–1384
Barros DO, Xavier SM, Barbosa CO, Silva RF, Maia DF, Oliveira AA, Freitas RM (2007) Efeitos da vitamina E da catalase de hipocampo após estado epiléptico induzido pela pilocarpina em ratos Wistar. Neurosci Lett 416:227–230
Ferreira PMP, Carvalho AFFU, Sousa DF, Magalhães JF, Martins AR, Martins MAC, Queiroz MGR (2007) Water extract of Moringa oleifera seeds: a toxicological approach. Rev Eletr Pesq Med 1:45–57
Freitas RM, Silva FO, Silva MGV, Feng D (2011) Antioxidant mechanisms of iso-6-cassine in suppressing seizures induced by pilocarpine. Rev Bras Farmacogn 21:437–443
Costa JP, Ferreira PB, De Sousa DP, Jordan J, Freitas RM (2012) Anticonvulsant effect of phytol in a pilocarpine model in mice. Neurosci Lett 523:115–118
De Sousa DP (2011) Analgesic-like activity of essential oils constituents. Molecules 16:2233–2252
Almeida AAC, Costa JP, Carvalho RBF, De Sousa DP, Freitas RM (2012) Evaluation of acute toxicity of a natural compound (+)-limonene epoxide and its anxiolytic-like action. Brain Res 1448:56–62
Campelo LML, Gonçalves FCM, Feitosa CM, Freitas RM (2011) Antioxidant activity of Citrus limon essential oil in mouse hippocampus. Pharm Biol 49:709–715
Bagamboula CF, Uyttendaele M, Debevere J (2004) Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and Shigellaflexneri. Food Microbiol 21:33–42
Botelho MA, Nogueira NA, Bastos GM, Fonseca SG, Lemos TL, Matos FJ, Montenegro D, Heukelbach J, Rao VS, Brito GA (2007) Antimicrobial activity of the essential oil from Lippia sidoides, carvacrol and thymol against oral pathogens. Braz J Med Biol Res 40:349–356
Klopell FC, Lemos M, Sousa JP, Comunello E, Maistro EL, Bastos JK, Andrade SF (2007) Nerolidol, an antiulcer constituent from the essential oil of Baccharis dracunculifolia DC (Asteraceae). Z Naturforsch 62:537–542
Pacifico S, D’Abrosca B, Golino A, Mastellone C, Piccolella S, Fiorentino A, Monaco P (2008) Antioxidant evaluation of polyhydroxylated nerolidols from redroot pigweed (Amaranthus retroflexus) leaves. LWT Food Sci Technol 41:1665–1671
Koudou J, Abena AA, Ngaissona P, Bessiére JM (2005) Chemical composition and pharmacological activity of essential oil of Canarium schweinfurthii. Fitoterapia 76:700–703
IFRA (International Fragrance Association) (2004) Use level survey
IFRA (International Fragrance Association) (2007) Volume of use survey
Motai T, Kitanaka S (2006) Sesquiterpenoids from Ferula fukanensis and their inhibitory effects on nitric oxide production. J Nat Med 60:54–57
Nogueira Neto JD, Almeida AAC, Silva OA, Carvalho RBF, De Sousa DP, Freitas RM (2012) Avaliação da toxicidade aguda e das propriedades ansiolíticas do nerolidol em camundongos. Biofar Revista de Biologia e Farmácia 8:42–55
Péres VF, Moura DJ, Sperotto ARM, Damasceno FC, Caramão EB, Zini CA, Saffi J (2009) Chemical composition and cytotoxic, mutagenic and genotoxic activities of the essential oil from Piper gaudichaudianum Kunth leaves. Food Chem Toxicol 47:2389–2395
Nogueira Neto JD, De Sousa DP, Freitas RM (2013) Evaluation of antioxidant potential in vitro of nerolidol. J Basic Appl Pharma Sci 34:125–130
Silva APS, Cerqueira GS, Nunes LCC, Freitas RM (2012) Effects of an aqueous extract of Orbignya phalerata Mart on locomotor activity and motor coordination in mice and as antioxidant in vitro. Pharmazie 67:260–263
Fortes AC, Almeida AAC, Mendonça-Júnior FJB, Freitas RM, Soares Sobrinho JL, Soares MFR (2013) Anxiolytic properties of new chemical entity, 5TIO1. Neurochem Res 38:726–731
Draper HH, Hadley M (1990) Malondialdehyde determination as an index of lipid peroxidation. Meth Enzymol 186:421–431
Freitas RLM, Santos IMS, Souza GF, Saldanha GB, Tomé AR, Freitas RM (2010) Oxidative stress in rat hippocampus caused by pilocarpine-induced seizures is reversed by buspirone. Brain Res Bull 81:505–509
Green LC, Tannenbaum SR, Goldman P (1981) Nitrate synthesis in the germfree and conventional rat. Science 212:56–58
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Flohé L, Ötting F (1984) Superoxide dismutase assays. Meth Enzymol 105:93–104
Freitas RM (2009) Investigation of oxidative stress involvement in hippocampus in epilepsy model induced by pilocarpine. Neurosc Lett 462:225–229
Chance B, Maehly AC (1955) Assay catalases and peroxidases. Meth Enzymol 2:764–768
Santos IMS, Tomé AR, Saldanha GB, Ferreira PMP, Militao GCG, Freitas RM (2009) Oxidative stress in the hippocampus during experimental seizures can be ameliorated with the antioxidant ascorbic acid. Oxid Med Cell Longev 2:1–8
Santos PS, Costa JP, Tomé AR, Saldanha GB, Souza GF, Feng D, Freitas RM (2011) Oxidative stress in rat striatum after pilocarpine-induced seizures is diminished by alpha-tocopherol. Eur J Pharmacol 668:65–71
Lapczynski A, Bhatia SP, Letizia CS, Api AM (2008) Fragrance material review on nerolidol (isomer unspecified). Food ChemToxicol 46:S247–S250
Bakkali F, Averbeck D, Idaomar M (2008) Biological effects of essential oils. Food Chem Toxicol 46:446–475
Arruda DC, D’alexandri FL, Katzin AM, Uliana SRB (2005) Antileishmanial activity of the terpene nerolidol. Antimicrob Agents Chemother 49:1679–1687
Lee SJ, Han JI, Lee GS, Park MJ, Choi IG, Na KJ, Jeung EB (2007) Antifungal effect of eugenol and nerolidol against Microsporum gypseum in a guinea pig model. Biol Pharm Bull 30:184–188
Roussis V, Chinou IB, Tsitsimpikou C, Vagias C, Petrakis PV (2001) Antibacterial activity of volatile secondary metabolites from caribbean soft corals of the genus Gorgonia. Flavour Fragr J 16:364–366
Canalle R, Burim RV, Lopes JLC, Takahashi CS (2001) Assessment of the cytotoxic and clastogenic activities os the sesquiterpene lactone lychnopholide in mammalian cells in vitro and in vivo. Cancer Detect Prev 25:93–101
Pículo F, Guiraldeli MC, Andrade SF, Luis ME (2011) In vivo genotoxicity assessment of nerolidol. J Appl Toxicol 7:633–639
Hollis L, Jones RS (2009) US Environmental Protection Agency Office of Pesticide Programs. Biopesticides and Pollution Prevention Division, Farnesol Nerolidol 1:24
Johri A, Beal FM (2012) Antioxidants in Huntington’s disease. Biochim Biophys Acta 1822:664–674
Greenough MA, Camakaris J, Bush AI (2013) Metal dyshomeostasis and oxidative stress in Alzheimer’s disease. Neurochem Int 62:540–555
Chirayu D, Pandya KR, Howell AP (2012) Antioxidants as potential therapeutics for neuropsychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry (in press)
Mecocci P, Polidori MP (2012) Antioxidant clinical trials in mild cognitive impairment and Alzheimer’s disease. Biochim Biophys Acta 1822:631–638
Kim D, Lee C (2004) Comprehensive study on vitamin C equivalent antioxidant capacity (VCEAC) of various polyphenols in scavenging a free radical and its structural relationship. Crit Rev Food Sci Nutr 44:253–273
Tiburcio JEV, Hoyos LAC, Asquieri ER (2010) Antocianinas, ácido ascórbico, polifenoles totales y actividad antioxidante, em la Cáscara de camu–camu (Myrciaria dubia (H.B.K) McVaugh). Ciênc Tecnol Aliment 30:151–160
Kim DO, Lee WK, Lee HJ, Lee CY (2002) Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J Agr Food Chem 50:3713–3717
Janowska B, Kurpios-Piec D, Prorok P, Szparecki G, Komisarski M, Kowalczyk P, Janion C, Tudek B (2012) Role of damage-specific DNA polymerases in M13 phage mutagenesis induced by a major lipid peroxidation product trans-4-hydroxy-2-nonenal. Mutat Res 29:41–51
Raedschelders K, Ansley DM, Chen DDY (2012) The cellular and molecular origin of reactive oxygen species generation during myocardial ischemia and reperfusion. Pharmacol Therap 133:230–255
Halliwell B, Gutteridge JCM (1999) Free radicals in biology and medicine, 3rd edn. Oxford Science Publications, London
Vanhatalo S, Riikonen R (2001) Nitric oxide metabolites, nitrates and nitrites in the cerebrospinal fluid in children with west syndrome. Epilepsy Res 46:3–13
Yoshida WB, Tardini DMS (2003) Brain injury due to ischemia and reperfusion in carotid edndarterectomy surgery. J Vasc Br 2:119–128
Michiels C, Raes M, Toussaint O, Remacle J (1994) Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free Radic Biol Med 17:235–248
Meister A (1995) Glutathione biosynthesis and its inhibition. Meth Enzymol 252:26–30
Simonié A, Laginja J, Varljen J, Zupan G, Erakovié V (2000) Lithium plus pilocarpine induced status epilepticus—biochemical changes. Neurosci Res 36:157–166
Naffah-Mazzacoratti MG, Cavalheiro EA, Ferreira EC, Abdalla DSP, Amado D, Bellissimo MI (2001) Pilocarpine-induced status epilepticus increases glutamate release in rat hippocampal synaptosomes. Epilepsy Res 46:121–128
Pong K, Yongqi Y, Doctrow SR, Baudry M (2002) Attenuation zinc-induced intracellular dysfunction and neurotoxicity by a synthetic superoxide dismutase/catalase mimetic, in cultured cortical neurons. Brain Res 950:218–230
Halliwell B, Gutteridge JMC (1992) Free radicals, antioxidants and human diseases: where are we now? J Lab Clin Med 119:598–620
Rauca C, Wiswedel I, Zerbe R, Keilhoff G, Krug M (2004) The role of superoxide dismutase and α-tocopherol in the development of seizures and kindling induced by pentylenetetrazol-influence of the radical scavenger a-phenyl-N-tert-butyl nitrone. Brain Res 1009:203–212
Nasuti C, Falcioni ML, Nwankwo LE, Cantalamessa F, Gabbianelli R (2008) Effect of permethrin plus antioxidants on locomotor activity and striatum in adolescent rats. Toxicol 251:45–50
Correa M, Sanchis-Segura C, Aragon CM (2001) Brain catalase activity is highly correlated with ethanol-induced locomotor activity in mice. Physiol Behav 73:641–647
Acknowledgments
This work was supported in part by Grants from the Brazilian National Research Council (CNPq), Brazil. R.M.F and P.S.S. are fellows from CNPq.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nogueira Neto, J.D., de Almeida, A.A.C., da Silva Oliveira, J. et al. Antioxidant Effects of Nerolidol in Mice Hippocampus After Open Field Test. Neurochem Res 38, 1861–1870 (2013). https://doi.org/10.1007/s11064-013-1092-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-013-1092-2