Abstract
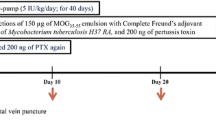
Gonadotrophin-releasing hormone (GnRH), a well known hypothalamic neuropeptide, has been reported to possess neurotrophic properties. Leuprolide acetate, a synthetic analogue of GnRH is considered to be a very safe and tolerable drug and it has been used for diverse clinical applications, including the treatment of prostate cancer, endometriosis, uterine fibroids, central precocious puberty and in vitro fertilization techniques. The present study was designed to determine whether Leuprolide acetate administration, exerts neurotrophic effects on clinical signs, body weight gain, neurofilaments (NFs) and myelin basic protein (MBP) expression, axonal morphometry and cell infiltration in spinal cord of experimental autoimmune encephalomyelitis (EAE) rats. In this work, we have found that Leuprolide acetate treatment decreases the severity of clinical signs of locomotion, induces a significantly greater body weight gain, increases the MBP and NFs expression, axonal area and cell infiltration in EAE animals. These results suggest the use of this agonist as a potential therapeutic approach for multiple sclerosis.







Similar content being viewed by others
References
Sospedra M, Martin R (2005) Immunology of multiple sclerosis. Annu Rev Immunol 23:683–747
O’Connor KC, Chitnis T, Griffin DE, Piyasirisilp S, Bar-Or A, Khoury S, Wucherpfennig KW, Hafler DA (2003) Myelin basic protein-reactive autoantibodies in the serum and cerebrospinal fluid of multiple sclerosis patients are characterized by low-affinity interactions. J Neuroimmunol 136:140–148
Sun D, Newman TA, Perry VH, Weller RO (2004) Cytokine-induced enhancement of autoimmune inflammation in the brain and spinal cord: implications for multiple sclerosis. Neuropathol Appl Neurobiol 30:374–384
Goverman J (2009) Autoimmune T cell responses in the central nervous system. Nat Rev Immunol 9:393–407
Fugger L, Friese MA, Bell JI (2009) From genes to function: the next challenge to understanding multiple sclerosis. Nat Rev Immunol 9:408–417
Baxter AG (2007) The origin and application of experimental autoimmune encephalomyelitis. Nat Rev Immunol 7:904–912
Sullivan AM, Toulouse A (2011) Neurotrophic factors for the treatment of Parkinson’s disease. Cytokine Growth Factor Rev 22:157–165
Thoenen H (1995) Neurotrophins and neuronal plasticity. Science 270:593–598
Barde YA (1997) Help from within for damaged axons. Nature 385:391–393
Moalem G, Gdalyahu A, Shani Y, Otten U, Lazarovici P, Cohen IR, Schwartz M (2000) Production of neurotrophins by activated T cells: implications for neuroprotective autoimmunity. J Autoimmun 15:331–345
Makar TK, Trisler D, Sura KT, Sultana S, Patel N, Bever CT (2008) Brain derived neurotrophic factor treatment reduces inflammation and apoptosis in experimental allergic encephalomyelitis. J Neurol Sci 270:70–76
Arnon R, Aharoni R (2009) Neuroprotection and neurogeneration in MS and its animal model EAE effected by glatiramer acetate. J Neural Transm 116:1443–1449
Lu Z, Hu X, Zhu C, Wang D, Zheng X, Liu Q (2009) Overexpression of CNTF in mesenchymal stem cells reduces demyelination and induces clinical recovery in experimental autoimmune encephalomyelitis mice. J Neuroimmunol 206:58–69
Prange-Kiel J, Jarry H, Schoen M, Kohlmann P, Lohse C, Zhou L, Rune GM (2008) Gonadotropin-releasing hormone regulates spine density via its regulatory role in hippocampal estrogen synthesis. J Cell Biol 180:417–426
Quintanar JL, Salinas E (2008) Neurotrophic effects of GnRH on neurite outgrowth and neurofilament protein expression in cultured cerebral cortical neurons of rat embryos. Neurochem Res 33:1051–1056
Liu Q, Xie F, Alvarado-Diaz A, Smith MA, Moreira PI, Zhu X, Perry G (2011) Neurofilamentopathy in neurodegenerative diseases. Open Neurol J 5:58–62
Perrot R, Berges R, Bocquet A, Eyer J (2008) Review of the multiple aspects of neurofilament functions, and their possible contribution to neurodegeneration. Mol Neurobiol 38:27–65
Quintanar JL, Salinas E, González R (2009) Gonadotropin-releasing hormone receptor in spinal cord neurons of embryos and adult rats. Neurosci Lett 461:21–24
Quintanar JL, Salinas E, Quintanar-Stephano A (2011) Gonadotropin-releasing hormone reduces the severity of experimental autoimmune encephalomyelitis, a model of multiple sclerosis. Neuropeptides 45:43–48
Wilson AC, Meethal SV, Bowen RL, Atwood CS (2007) Leuprolide acetate: a drug of diverse clinical applications. Expert Opin Investig Drugs 16:1851–1863
Fujino M, Fukuda T, Shinagawa S, Kobayashi S, Yamazaki I (1974) Synthetic analogs of luteinizing hormone releasing hormone (LH-RH) substituted in position 6 and 10. Biochem Biophys Res Commun 60:406–413
Abouelfadel Z, Crawford ED (2008) Leuprorelin depot injection: patient considerations in the management of prostatic cancer. Ther Clin Risk Manag 4:513–526
Wise PM (2002) Estrogens and neuroprotection. Trends Endocrinol Metab 13:229–230
Vadakkadath Meethal SV, Atwood CS (2005) The role of hypothalamic-pituitary-gonadal hormones in the normal structure and functioning of the brain. Cell Mol Life Sci 62:257–270
Quintanar-Stephano A, Chavira-Ramírez R, Kovacs K, Berczi I (2005) Neurointermediate pituitary lobectomy decreases the incidence and severity of experimental autoimmune encephalomyelitis in Lewis rats. J Endocrinol 184:51–58
Becher B, Durell BG, Miga AV, Hickey WF, Noelle RJ (2001) The clinical course of experimental autoimmune encephalomyelitis and inflammation is controlled by the expression of CD40 within the central nervous system. J Exp Med 193:967–974
Rossi S, Furlan R, De Chiara V, Motta C, Studer V, Mori F, Musella A, Bergami A, Muzio L, Bernardi G, Battistini L, Martino G, Centonze D (2012) Interleukin-1β causes synaptic hyperexcitability in multiple sclerosis. Ann Neurol 71:76–83
Pollak Y, Orion E, Goshen I, Ovadia H, Yirmiya R (2002) Experimental autoimmune encephalomyelitis-associated behavioral syndrome as a model of ‘depression due to multiple sclerosis’. Brain Behav Immun 16:533–543
Lou ZY, Chen C, He Q, Zhao CB, Xiao BG (2011) Targeting CB2 receptor as a neuroinflammatory modulator in experimental autoimmune encephalomyelitis. Mol Immunol 49:453–461
Vana AC, Li S, Ribeiro R, Tchantchou F, Zhang Y (2011) Arachidonyl trifluoromethyl ketone ameliorates experimental autoimmune encephalomyelitis via blocking peroxynitrite formation in mouse spinal cord white matter. Exp Neurol 231:45–55
Smith MR, Lee H, Fallon MA, Nathan DM (2008) Adipocytokines, obesity, and insulin resistance during combined androgen blockade for prostate cancer. Urology 71:318–322
Harris VK, Sadiq SA (2009) Disease biomarkers in multiple sclerosis, potential for use in therapeutic decision making. Mol Diagn Ther 13:225–244
Gresle MM, Butzkueven H, Shaw G (2011) Neurofilament proteins as body fluid biomarkers of neurodegeneration in multiple sclerosis. Mult Scler Int 2011:1–7
Vickers JC, King AE, Woodhouse A, Kirkcaldie MT, Staal JA, McCormack GH, Blizzard CA, Musgrove RE, Mitew S, Liu Y, Chuckowree JA, Bibari O, Dickson TC (2009) Axonopathy and cytoskeletal disruption in degenerative diseases of the central nervous system. Brain Res Bull 80:217–223
Avsar T, Korkmaz D, Tütüncü M, Demirci NO, Saip S, Kamasak M, Siva A, Turanli ET (2012) Protein biomarkers for multiple sclerosis: semi-quantitative analysis of cerebrospinal fluid candidate protein biomarkers in different forms of multiple sclerosis. Mult Scler. doi:10.1177/1352458511433303
De Rosbo NK, Bernard CCA, Simmons RD, Carnegie PR (1985) Concomitant detection of changes in myelin basic protein and permeability of blood-spinal cord barrier in acute experimental autoimmune encephalomyelitis by electroimmunoblotting. J Neuroimmunol 9:349–361
Almolda B, Costa M, Montoya M, González B, Castellano B (2011) Increase in Th17 and T-reg lymphocytes and decrease of IL22 correlate with the recovery phase of acute EAE in rat. PLoS One 6:e27473
Mirowska-Guzel D (2009) The role of neurotrophic factors in the pathology and treatment of multiple sclerosis. Immunopharmacol Immunotoxicol 31:32–38
Kastin AJ, Coy DH, Schally AV, Zadina JE (1980) Dissociation of effects of LH-RH analogs on pituitary regulation and reproductive behavior. Pharmacol Biochem Behav 13:913–914
Barrera CM, Kastin AJ, Fasold MB, Banks WA (1991) Bidirectional saturable transport of LHRH across the blood-brain barrier. Am J Physiol 261:E312–E318
Acknowledgments
We would like to express our sincere gratitude to Dr. Kalman Kovacs for reviewing the manuscript and to Dr. Andrés Quintanar-Stephano and Lic. Denisse Calderón Vallejo for the methodological support. We thank to the Consejo Nacional de Ciencia y Tecnología (CONACyT) for scholarship 266788.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guzmán-Soto, I., Salinas, E., Hernández-Jasso, I. et al. Leuprolide Acetate, a GnRH Agonist, Improves Experimental Autoimmune Encephalomyelitis: A Possible Therapy for Multiple Sclerosis. Neurochem Res 37, 2190–2197 (2012). https://doi.org/10.1007/s11064-012-0842-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-012-0842-x