Abstract
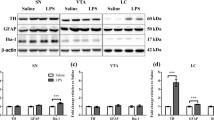
Tyrosine hydroxylase (TH) is the rate-limiting enzyme in the biosynthetic pathway for catecholamine synthesis. Stress triggers an increase in TH activity, resulting in increased release of catecholamines from both neurons and the adrenal medulla. In response to stress three phases of TH activation have been identified (acute, sustained and chronic) and each phase has a unique mechanism. The acute and chronic phases have been studied in vivo in a number of animal models, but to date the sustained phase has only been characterised in vitro. We aimed to investigate the effects of dual exposure to lipopolysaccharide (LPS) in neonatal rats on TH protein, TH phosphorylation at serine residues 19, 31 and 40 and TH activity in the adrenal gland over the sustained phase. Wistar rats were administered LPS (0.05 mg/kg, intraperitoneal injection) or an equivolume of non-pyrogenic saline on days 3 and 5 postpartum. Adrenal glands were collected at 4, 24 and 48 h after the drug exposure on day 5. Neonatal LPS treatment resulted in increases in TH phosphorylation of Ser40 at 4 and 24 h, TH phosphorylation of Ser31 at 24 h, TH activity at 4 and 24 h and TH protein at 48 h. We therefore have provided evidence for the first time that TH phosphorylation at Ser31 and Ser40 occurs for up to 24 h in vivo and leads to TH activation independent of TH protein synthesis, suggesting that the sustained phase of TH activation occurs in vivo.


Similar content being viewed by others
Abbreviations
- 2DG:
-
2-deoxy-d-glucose
- LPS:
-
Lipopolysaccharide
- PND:
-
Postnatal day
- Ser:
-
Serine residue
- TBST:
-
Tris-buffered saline with Tween
- TH:
-
Tyrosine hydroxylase,
References
Axelrod J, Reisine TD (1984) Stress hormones: their interaction and regulation. Science 224(4648):452–459
Flatmark T (2000) Catecholamine biosynthesis and physiological regulation in neuroendocrine cells. Acta Physiol Scand 168(1):1–17
Zigmond RE, Schwarzschild MA, Rittenhouse AR (1989) Acute regulation of tyrosine hydroxylase by nerve activity and by neurotransmitters via phosphorylation. Annu Rev Neurosci 12:415–461. doi:10.1146/annurev.ne.12.030189.002215
Wakade AR, Wakade TD, Malhotra RK (1988) Restoration of catecholamine content of previously depleted adrenal medulla in vitro: importance of synthesis in maintaining the catecholamine stores. J Neurochem 51(3):820–829
Nagatsu T, Levitt M, Udenfriend S (1964) Tyrosine hydroxylase. the initial step in norepinephrine biosynthesis. J Biol Chem 239:2910–2917
Kumer SC, Vrana KE (1996) Intricate regulation of tyrosine hydroxylase activity and gene expression. J Neurochem 67(2):443–462
Ong LK, Guan L, Stutz B, Dickson PW, Dunkley PR, Bobrovskaya L (2011) The effects of footshock and immobilization stress on tyrosine hydroxylase phosphorylation in the rat locus coeruleus and adrenal gland. Neuroscience 192:20–27. doi:10.1016/j.neuroscience.2011.06.087
Bobrovskaya L, Damanhuri HA, Ong LK, Schneider JJ, Dickson PW, Dunkley PR, Goodchild AK (2010) Signal transduction pathways and tyrosine hydroxylase regulation in the adrenal medulla following glucoprivation: an in vivo analysis. Neurochem Int 57(2):162–167. doi:10.1016/j.neuint.2010.05.009
Ong LK, Bobrovskaya L, Walker FR, Day TA, Dickson PW, Dunkley PR (2011) The effect of social defeat on tyrosine hydroxylase phosphorylation in the rat brain and adrenal gland. Neurochem Res 36(1):27–33. doi:10.1007/s11064-010-0255-7
Sabban EL, Kvetnansky R (2001) Stress-triggered activation of gene expression in catecholaminergic systems: dynamics of transcriptional events. Trends Neurosci 24(2):91–98
Bobrovskaya L, Gilligan C, Bolster EK, Flaherty JJ, Dickson PW, Dunkley PR (2007) Sustained phosphorylation of tyrosine hydroxylase at serine 40: a novel mechanism for maintenance of catecholamine synthesis. J Neurochem 100(2):479–489. doi:10.1111/j.1471-4159.2006.04213.x
Bobrovskaya L, Gelain DP, Gilligan C, Dickson PW, Dunkley PR (2007) PACAP stimulates the sustained phosphorylation of tyrosine hydroxylase at serine 40. Cell Signal 19(6):1141–1149. doi:10.1016/j.cellsig.2006.12.006
Kvetnansky R, Sabban EL, Palkovits M (2009) Catecholaminergic systems in stress: structural and molecular genetic approaches. Physiol Rev 89(2):535–606. doi:10.1152/physrev.00042.2006
Wong DL, Tank AW (2007) Stress-induced catecholaminergic function: transcriptional and post-transcriptional control. Stress 10(2):121–130. doi:10.1080/10253890701393529
Tank AW, Xu L, Chen X, Radcliffe P, Sterling CR (2008) Post-transcriptional regulation of tyrosine hydroxylase expression in adrenal medulla and brain. Ann N Y Acad Sci 1148:238–248. doi:10.1196/annals.1410.054
Sominsky L, Walker AK, Ong LK, Tynan RJ, Walker FR, Hodgson DM (2012) Increased microglial activation in the rat brain following neonatal exposure to a bacterial mimetic. Behav Brain Res 226(1):351–356. doi:10.1016/j.bbr.2011.08.038
Walker AK, Hiles SA, Sominsky L, McLaughlin EA, Hodgson DM (2011) Neonatal lipopolysaccharide exposure impairs sexual development and reproductive success in the Wistar rat. Brain Behav Immun 25(4):674–684. doi:10.1016/j.bbi.2011.01.004
Walker AK, Nakamura T, Byrne RJ, Naicker S, Tynan RJ, Hunter M, Hodgson DM (2009) Neonatal lipopolysaccharide and adult stress exposure predisposes rats to anxiety-like behaviour and blunted corticosterone responses: implications for the double-hit hypothesis. Psychoneuroendocrinology 34(10):1515–1525. doi:10.1016/j.psyneuen.2009.05.010
Walker AK, Nakamura T, Hodgson DM (2010) Neonatal lipopolysaccharide exposure alters central cytokine responses to stress in adulthood in Wistar rats. Stress 13(6):506–515. doi:10.3109/10253890.2010.489977
Shanks N, Larocque S, Meaney MJ (1995) Neonatal endotoxin exposure alters the development of the hypothalamic-pituitary-adrenal axis: early illness and later responsivity to stress. J Neurosci Off J Soc Neurosci 15(1 Pt 1):376–384
Gordon SL, Bobrovskaya L, Dunkley PR, Dickson PW (2009) Differential regulation of human tyrosine hydroxylase isoforms 1 and 2 in situ: isoform 2 is not phosphorylated at Ser35. Biochim Biophys Acta 1793(12):1860–1867. doi:10.1016/j.bbamcr.2009.10.001
Briggs GD, Gordon SL, Dickson PW (2011) Mutational analysis of catecholamine binding in tyrosine hydroxylase. Biochemistry 50(9):1545–1555. doi:10.1021/bi101455b
Haycock JW (1993) Multiple signaling pathways in bovine chromaffin cells regulate tyrosine hydroxylase phosphorylation at Ser19, Ser31, and Ser40. Neurochem Res 18(1):15–26
Dunkley PR, Bobrovskaya L, Graham ME, von Nagy-Felsobuki EI, Dickson PW (2004) Tyrosine hydroxylase phosphorylation: regulation and consequences. J Neurochem 91(5):1025–1043. doi:10.1111/j.1471-4159.2004.02797.x
Craviso GL, Hemelt VB, Waymire JC (1992) Nicotinic cholinergic regulation of tyrosine hydroxylase gene expression and catecholamine synthesis in isolated bovine adrenal chromaffin cells. J Neurochem 59(6):2285–2296
Watanabe Y, McKittrick CR, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR (1995) Effects of chronic social stress on tyrosine hydroxylase mRNA and protein levels. Brain Res Mol Brain Res 32(1):176–180
Rusnak M, Jelokova J, Vietor I, Sabban EL, Kvetnansky R (1998) Different effects of insulin and 2-deoxy-d-glucose administration on tyrosine hydroxylase gene expression in the locus coeruleus and the adrenal medulla in rats. Brain Res Bull 46(5):447–452
Tank AW, Meligeni J, Weiner N (1984) Cyclic AMP-dependent protein kinase is not involved in the in vivo activation of tyrosine hydroxylase in the adrenal gland after decapitation. J Biol Chem 259(14):9269–9276
Nankova B, Kvetnansky R, McMahon A, Viskupic E, Hiremagalur B, Frankle G, Fukuhara K, Kopin IJ, Sabban EL (1994) Induction of tyrosine hydroxylase gene expression by a nonneuronal nonpituitary-mediated mechanism in immobilization stress. Proc Natl Acad Sci U S A 91(13):5937–5941
Baruchin A, Weisberg EP, Miner LL, Ennis D, Nisenbaum LK, Naylor E, Stricker EM, Zigmond MJ, Kaplan BB (1990) Effects of cold exposure on rat adrenal tyrosine hydroxylase: an analysis of RNA, protein, enzyme activity, and cofactor levels. J Neurochem 54(5):1769–1775
Fluharty SJ, Snyder GL, Stricker EM, Zigmond MJ (1983) Short- and long-term changes in adrenal tyrosine hydroxylase activity during insulin-induced hypoglycemia and cold stress. Brain Res 267(2):384–387
Jedynak JP, Ali SF, Haycock JW, Hope BT (2002) Acute administration of cocaine regulates the phosphorylation of serine-19, -31 and -40 in tyrosine hydroxylase. J Neurochem 82(2):382–388
Nunez C, Laorden ML, Milanes MV (2007) Regulation of serine (Ser)-31 and Ser40 tyrosine hydroxylase phosphorylation during morphine withdrawal in the hypothalamic paraventricular nucleus and nucleus tractus solitarius-A2 cell group: role of ERK1/2. Endocrinology 148(12):5780–5793. doi:10.1210/en.2007-0510
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia, Hunter Medical Research Institute and University of Newcastle, Australia.
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ong, L.K., Sominsky, L., Dickson, P.W. et al. The Sustained Phase of Tyrosine Hydroxylase Activation In vivo. Neurochem Res 37, 1938–1943 (2012). https://doi.org/10.1007/s11064-012-0812-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-012-0812-3