Abstract
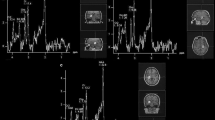
This study aims to investigate the retinal metabolic processes in a rat axotomy model. Retinal metabolic changes in optic nerve transection (ONT) rat model were analyzed by 1H magnetic resonance spectroscopy (1H-MRS). Retinal ganglion cells (RGCs) densities were assessed from retinal whole mounts. The retina was stained immunohistochemically with glial fibrillary acidic protein (GFAP). The results showed that the retina in ONT rats had significantly decreased concentrations of γ-aminobutyric acid (GABA), N-acetylaspartate (NAA), taurine (Tau), creatine (Cr) and increased concentrations of alanine (Ala) compared with control. Examination of glutamate (Glu), glutamine (Gln) and Glx (Glu + Gln) concentrations disclosed no significant differences. The mean density of RGCs reduced from 2,249 ± 87 cells/mm2 in control group to 320 ± 56 cells/mm2 in ONT group. GFAP immunoreactivity was markedly higher in ONT group than that in control group. The retinal metabolism after ONT was associated with neurotransmitter recycling/production perturbation, as well as other metabolic disequilibrium.



Similar content being viewed by others
References
Kermer P, Klocker N, Weishaupt JH et al (2001) Transection of the optic nerve in rats: studying neuronal death and survival in vivo. Brain Res Brain Res Protoc 7:255–260
Munemasa Y, Kim SH, Ahn JH et al (2008) Protective effect of thioredoxins 1 and 2 in retinal ganglion cells after optic nerve transection and oxidative stress. Invest Ophthalmol Vis Sci 49:3535–3543
Vorwerk CK, Lipton SA, Zurakowski D et al (1996) Chronic low-dose glutamate is toxic to retinal ganglion cells. Toxicity blocked by memantine. Invest Ophthalmol Vis Sci 37:1618–1624
Eitan S, Solomon A, Lavie V et al (1994) Recovery of visual response of injured adult rat optic nerves treated with transglutaminase. Science 264:1764–1768
Lieven CJ, Hoegger MJ, Schlieve CR et al (2006) Retinal ganglion cell axotomy induces an increase in intracellular superoxide anion. Invest Ophthalmol Vis Sci 47:1477–1485
Nguyen SM, Alexejun CN, Levin LA (2003) Amplification of a reactive oxygen species signal in axotomized retinal ganglion cells. Antioxid Redox Signal 5:629–634
Weishaupt JH, Bahr M (2001) Degeneration of axotomized retinal ganglion cells as a model for neuronal apoptosis in the central nervous system: molecular death and survival pathways. Restor Neurol Neurosci 19:19–27
Li CX, Wang Y, Gao H et al (2008) Cerebral metabolic changes in a depression-like rat model of chronic forced swimming studied by ex vivo high resolution 1H magnetic resonance spectroscopy. Neurochem Res 33:2342–2349
Gao H, Xiang Y, Sun N et al (2007) Metabolic changes in rat prefrontal cortex and hippocampus induced by chronic morphine treatment studied ex vivo by high resolution 1H NMR spectroscopy. Neurochem Int 50:386–394
Pears MR, Cooper JD, Mitchison HM et al (2005) High resolution 1H NMR-based metabolomics indicates a neurotransmitter cycling deficit in cerebral tissue from a mouse model of Batten disease. J Biol Chem 280:42508–42514
Somorjai RL, Dolenko B, Nikulin AK et al (1996) Classification of 1H MR spectra of human brain neoplasms: the influence of preprocessing and computerized consensus diagnosis on classification accuracy. J Magn Reson Imag 6:437–444
Bell JD, Lee JA, Lee HA et al (1991) Nuclear magnetic resonance studies of blood plasma and urine from subjects with chronic renal failure: identification of trimethylamine-N-oxide. Biochim Biophys Acta 1096:101–107
Gribbestad IS, Midelfart A (1994) High-resolution 1H NMR spectroscopy of aqueous humour from rabbits. Graefes Arch Clin Exp Ophthalmol 232:494–498
Makoroff KL, Cecil KM, Care M et al (2005) Elevated lactate as an early marker of brain injury in inflicted traumatic brain injury. Pediatr Radiol 35:668–676
Hunter JV, Thornton RJ, Wang ZJ et al (2005) Late proton MR spectroscopy in children after traumatic brain injury: correlation with cognitive outcomes. AJNR Am J Neuroradiol 26:482–488
Ashwal S, Holshouser B, Tong K et al (2004) Proton MR spectroscopy detected glutamate/glutamine is increased in children with traumatic brain injury. J Neurotrauma 21:1539–1552
Farooque M, Hillered L, Holtz A et al (1996) Changes of extracellular levels of amino acids after graded compression trauma to the spinal cord: an experimental study in the rat using microdialysis. J Neurotrauma 13:537–548
Rango M, Spagnoli D, Tomei G et al (1995) Central nervous system trans-synaptic effects of acute axonal injury: a 1H magnetic resonance spectroscopy study. Magn Reson Med 33:595–600
Hashimoto M, Ohtsuka K, Harada K (2004) N-acetylaspartate concentration in the chiasm measured by in vivo proton magnetic resonance spectroscopy. Jpn J Ophthalmol 48:353–357
Ngumah QC, Buchthal SD, Dacheux RF (2006) Longitudinal non-invasive proton NMR spectroscopy measurement of vitreous lactate in a rabbit model of ocular hypertension. Exp Eye Res 83:390–400
Chan KC, So KF, Wu EX (2009) Proton magnetic resonance spectroscopy revealed choline reduction in the visual cortex in an experimental model of chronic glaucoma. Exp Eye Res 88:65–70
Cui Q, Yip HK, Zhao RC et al (2003) Intraocular elevation of cyclic AMP potentiates ciliary neurotrophic factor-induced regeneration of adult rat retinal ganglion cell axons. Mol Cell Neurosci 22:49–61
Grosskreutz CL, Hanninen VA, Pantcheva MB et al (2005) FK506 blocks activation of the intrinsic caspase cascade after optic nerve crush. Exp Eye Res 80:681–686
Villegas-Perez MP, Vidal-Sanz M, Rasminsky M et al (1993) Rapid and protracted phases of retinal ganglion cell loss follow axotomy in the optic nerve of adult rats. J Neurobiol 24:23–36
Xue LP, Lu J, Cao Q et al (2006) Nestin expression in Muller glial cells in postnatal rat retina and its upregulation following optic nerve transection. Neuroscience 143:117–127
Panagis L, Thanos S, Fischer D et al (2005) Unilateral optic nerve crush induces bilateral retinal glial cell proliferation. Eur J Neurosci 21:2305–2309
Lam DM (1997) Neurotransmitters in the vertebrate retina. Invest Ophthalmol Vis Sci 38:553–556
Yang XL (2004) Characterization of receptors for glutamate and GABA in retinal neurons. Prog Neurobiol 73:127–150
Thoreson WB, Witkovsky P (1999) Glutamate receptors and circuits in the vertebrate retina. Prog Retin Eye Res 18:765–810
Poitry S, Poitry-Yamate C, Ueberfeld J et al (2000) Mechanisms of glutamate metabolic signaling in retinal glial (Muller) cells. J Neurosci 20:1809–1821
Ramonet D, Rodríguez MJ, Fredriksson K et al (2004) In vivo neuroprotective adaptation of the glutamate/glutamine cycle to neuronal death. Hippocampus 14:586–594
Kielczewski JL, Pease ME, Quigley HA (2005) The effect of experimental glaucoma and optic nerve transection on amacrine cells in the rat retina. Invest Ophthalmol Vis Sci 46:3188–3196
Moffett JR, Ross B, Arun P et al (2007) N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol 81:89–131
Wharton BA, Morley R, Isaacs EB et al (2004) Low plasma taurine and later neurodevelopment. Arch Dis Child Fetal Neonatal Ed 89:F497–F498
Chen K, Zhang Q, Wang J et al (2009) Taurine protects transformed rat retinal ganglion cells from hypoxia-induced apoptosis by preventing mitochondrial dysfunction. Brain Res 1279:131–138
Saransaari P, Oja SS (2010) Modulation of taurine release in ischemia by glutamate receptors in mouse brain stem slices. Amino Acids 38:739–746
Pow DV, Sullivan R, Reye P et al (2002) Localization of taurine transporters, taurine, and (3) H taurine accumulation in the rat retina, pituitary, and brain. Glia 37:153–168
Wellard RM, Briellmann RS, Wilson JC et al (2004) Longitudinal study of MRS metabolites in Rasmussen encephalitis. Brain 127:1302–1312
Acknowledgments
This work was supported by grant 30700922 from National Natural Science Foundation of China.
Conflicts of interest
No potential conflicts of interest are disclosed.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors Shuang Li and Mingming Huang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, S., Huang, M., Wang, X. et al. Retinal Metabolic Changes in an Experimental Model of Optic Nerve Transection by Ex Vivo 1H Magnetic Resonance Spectroscopy. Neurochem Res 36, 2427–2433 (2011). https://doi.org/10.1007/s11064-011-0570-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-011-0570-7