Abstract
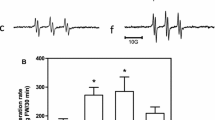
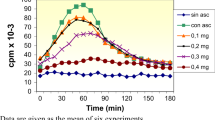
This study explored the effect of coral calcium hydride (CCH) on rat intrahippocampal antioxidant ability by measuring the PCAM nitroxide radical decay ratio when CCH was (a) co-perfused into the hippocampus and (b) fed orally to the rats for 4 weeks under a freely moving state. Estimation of the in vivo antioxidant effect was obtained by administration of the blood–brain barrier-permeable PCAM nitroxide radical and the measured PCAM radical decay ratio then correlated to the amount of antioxidant in the brain using electron spin resonance (ESR) spectroscopy combined with microdialysis. The half-life periods of PCAM in rats treated with CCH in both the co-perfusion and orally fed groups were significantly shorter compared to the control group. These results clarify the mechanism that CCH may exert antioxidant activity by significantly enhancing the basal endogenous antioxidant ability in the hippocampus through a synergistic effect with α-tocopherol and ascorbic acid.




Similar content being viewed by others
References
Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S (2007) Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med 13:688–694
Cai J, Kang Z, Liu WW, Luo X, Qiang S, Zhang JH, Ohta S, Sun X, Xu W, Tao H, Li R (2008) Hydrogen therapy reduces apoptosis in neonatal hypoxia-ischemia rat model. Neurosci Lett 441:167–172
Nagata K, Nakashima-Kamimura N, Mikami T, Ohsawa I, Ohta S (2009) Consumption of molecular hydrogen prevents the stress-induced impairments in hippocampus-dependent learning tasks during chronic physical restraint in mice. Neuropsychopharmacology 34:501–508
Ueda Y, Yokoyama H, Tokumaru J, Doi T, Nakajima A (2004) Kinetics of extracellular nitroxide radical and glutamate levels in the hippocampus of conscious rats: cautionary note to the application of nitroxide radical on clinical arena. Neurochem Res 29:1695–1701
Ueda Y, Doi T, Takaki M, Nagatomo K, Nakajima A, Willmore LJ (2009) Levetiracetam enhances endogenous antioxidant in the hippocampus of rats: in vivo evaluation by brain microdialysis combined with ESR spectroscopy. Brain Res 1266:1–7
Pellegrino JL, Pellegrino AS, Cushman AJA (1986) Stereotaxic atlas of the rat brain. Plenum Press, New York and London
Ueda Y, Yokoyama H, Ohya-Nishiguchi H, Kamada H (1998) ESR spectroscopy for analysis of hippocampal elimination of a nitroxide radical during kainic acid-induced seizure in rats. Magn Reson Med 40:491–493
Ueda Y, Doi T, Nagatomo K, Nakajima A (2007) Protective role of pentobarbital pretreatment for NMDA-R activated lipid peroxidation is derived from the synergistic effect on endogenous anti-oxidant in the hippocampus of rats. Neurosci Lett 417:46–49
Ueda Y, Doi T, Tokumaru J, Nakajima A, Nagatomo K (2005) In vivo evaluation of the effect of zonisamide on the hippocampal redox state during kainic acid-Induced seizure status in rats. Neurochem Res 30:1117–1121
Hiramatsu M, Takahashi T, Oikawa T (2007) Effect of food (Dr. ZP-O AH) generating hydrogen on lipid peroxide in the brain of senescence accelerated mice (SAM/P-8) and ddY mice. J Brain Sci 33:41
Tokumaru J, Ueda Y, Yokoyama H, Nakajima A, Doi T, Mitsuyama Y, Ohya-Nishiguchi H, Kamada H (2000) In vivo evaluation of hippocampal anti-oxidant ability of zonisamide in rats. Neurochem Res 25:1107–1111
Noor JI, Ikeda T, Ueda Y, Ikenoue T (2005) A free radical scavenger, edaravone, inhibits lipid peroxidation and the production of nitric oxide in hypoxic-ischemic brain damage of neonatal rats. Am J Obstet Gynecol 193:1703–1708
Jenner P, Olanow CW (1996) Oxidative stress and the pathogenesis of Parkinson’s disease. Neurology 47:S161–S170
Ueda Y, Doi T, Nagatomo K, Nakajima A (2007) In vivo activation of N-methyl-d-aspartate receptors generates free radicals and reduces antioxidant ability in the rat hippocampus: Experimental protocol of in vivo ESR spectroscopy and microdialysis for redox status evaluation. Brain Res 1178:20–27
Rao AK, Ziegler YS, McLeod IX, Yates JR, Nardulli AM (2009) Thioredoxin and thioredoxin reductase influence estrogen receptor alpha-mediated gene expression in human breast cancer cells. J Mol Endocrinol 43:251–261
Guldiken B, Demir M, Guldiken S, Turgut N, Turgut B, Tugrul A (2009) Oxidative stress and total antioxidant capacity in diabetic and nondiabetic acute ischemic stroke patients. Clin Appl Thromb Hemost 15:695–700
Acknowledgements
This study was partially supported by a Grant-in-Aid for Scientific Research (C) (2) (16591146, 18591297, and 20591372 to Y.U.) from the Ministry of Education, Science, Sports, and Culture, Japan. CCH was donated by ICB.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ueda, Y., Nakajima, A. & Oikawa, T. Hydrogen-Related Enhancement of In Vivo Antioxidant Ability in the Brain of Rats Fed Coral Calcium Hydride. Neurochem Res 35, 1510–1515 (2010). https://doi.org/10.1007/s11064-010-0204-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-010-0204-5