Abstract
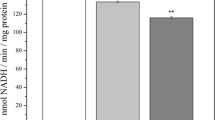
Malondialdehyde (MDA) is a product of oxidative damage to lipids, amino acids and DNA, and accumulates with aging and diseases. MDA can possibly react with amines so as to modify proteins and inactivate enzymes; it can also modify nucleosides so as to cause mutagenicity. Brain mitochondrial dysfunction is a major contributor to aging and neurodegenerative diseases. We hypothesize that MDA accumulated during aging targets mitochondrial enzymes so as to cause further mitochondrial dysfunction and additional contributions to aging and neurodegeneration. Herein, we investigated the neuronal mitochondrial toxic effects of MDA on mitochondrial respiration and activities of enzymes (mitochondrial complexes I–V, α-ketoglutarate dehydrogenase (KGDH) and pyruvate dehydrogenase (PDH)), in isolated rat brain mitochondria. MDA depressed mitochondrial membrane potential, and also showed a dose-dependent inhibition of mitochondrial complex I- and complex II-linked respiration. Complex I and II, and PDH activities were depressed by MDA at ≥0.2 μmol/mg; KGDH and complex V were inhibited by ≥0.4 and ≥1.6 μmol MDA/mg, respectively. However, MDA did not have any toxic effects on complex III and IV activities over the range 0–2 μmol/mg. MDA significantly elevated mitochondrial reactive oxygen species (ROS) and protein carbonyls at 0.2 and 0.002 μmol/mg, respectively. As for the antioxidant defense system, a high dose of MDA slightly decreased mitochondrial GSH and superoxide dismutase. These results demonstrate that MDA causes neuronal mitochondrial dysfunction by directly promoting generation of ROS and modifying mitochondrial proteins. The results suggest that MDA-induced neuronal mitochondrial toxicity may be an important contributing factor to brain aging and neurodegenerative diseases.








Similar content being viewed by others
References
Harman D (1961) Prolongation of the normal lifespan and inhibition of spontaneous cancer by antioxidants. J Gerontol 16:247–254
Floyd RA, West M, Hensley K (2001) Oxidative biochemical markers; clues to understanding aging in long- lived species. Exp Gerontol 36:619–640. doi:10.1016/S0531-5565(00)00231-X
Liu J, Mori A (1999) Stress, aging, and brain oxidative damage. Neurochem Res 24:1479–1497. doi:10.1023/A:1022597010078
Levine RL, Garland D, Oliver CN et al (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478. doi:10.1016/0076-6879(90)86141-H
Esterbauer H, Schaur RJ, Zollner H (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 11:81–128. doi:10.1016/0891-5849(91)90192-6
Gutteridge JMC (1981) Thiobarbituric acid-reactivity following iron-dependent free-radicals damage to amino acids and carbohydrates. FEBS Lett 128:343–346. doi:10.1016/0014-5793(81)80113-5
Burger RM, Berkowitz AR, Peisach J, Horwitz SB (1980) Origin of malondialdehyde from DNA degraded by Fe(II) x bleomycin. J Biol Chem 255:11832–11838
Cheeseman KH, Emery S, Maddix SP, Slater TF, Burton GW, Ingold KU (1988) Studies on lipid peroxidation in normal and tumour tissues. The Yoshida rat liver tumour. Biochem J 250:247–252
Liu J, Ames BN (2005) Reducing mitochondrial decay with mitochondrial nutrients to delay and treat cognitive dysfunction, Alzheimer’s disease, and Parkinson’s disease. Nutr Neurosci 8:67–89. doi:10.1080/10284150500047161
Liu J, Atamna H, Hirohiko K, Ames BN (2002) Delaying brain mitochondrial decay and aging with mitochondrial antioxidants and metabolites. Ann N Y Acad Sci 959:133–166
Picklo MJ, Amarnath V, McIntyre JO, Graham DG, Montine TJ (1999) 4-Hydroxy-2(E)-nonenal inhibits CNS mitochondrial respiration at multiple sites. J Neurochem 72:1617–1624. doi:10.1046/j.1471-4159.1999.721617.x
Humphries KM, Szweda LI (1998) Selective inactivation of alpha-ketoglutarate dehydrogenase and pyruvate dehydrogenase: reaction of lipoic acid with 4-hydroxy-2-nonenal. Biochemistry 37:15835–15841. doi:10.1021/bi981512h
Neely MD, Zimmerman L, Picklo MJ et al (2000) Congeners of N(alpha)-acetyl-l-cysteine but not aminoguanidine act as neuroprotectants from the lipid peroxidation product 4-hydroxy-2-nonenal. Free Radic Biol Med 29:1028–1036. doi:10.1016/S0891-5849(00)00411-1
Long J, Wang X, Gao H et al (2006) Malonaldehyde acts as a mitochondrial toxin: inhibitory effects on respiratory function and enzyme activities in isolated rat liver mitochondria. Life Sci 79:1466–1472. doi:10.1016/j.lfs.2006.04.024
Greilberger J, Koidl C, Greilberger M et al (2008) Malondialdehyde, carbonyl proteins and albumin-disulphide as useful oxidative markers in mild cognitive impairment and Alzheimer’s disease. Free Radic Res 42:633–638. doi:10.1080/10715760802255764
Bourdel-Marchasson I, Delmas-Beauvieux MC, Peuchant E et al (2001) Antioxidant defences and oxidative stress markers in erythrocytes and plasma from normally nourished elderly Alzheimer patients. Age Ageing 30:235–241. doi:10.1093/ageing/30.3.235
Nagai T, Yamada K, Kim HC et al (2003) Cognition impairment in the genetic model of aging klotho gene mutant mice: a role of oxidative stress. FASEB J 17:50–52
Zhou XM, Cao YL, Dou DQ (2006) Protective effect of ginsenoside-Re against cerebral ischemia/reperfusion damage in rats. Biol Pharm Bull 29:2502–2505. doi:10.1248/bpb.29.2502
Wang QL, Lin M, Liu GT (2001) Antioxidative activity of natural isorhapontigenin. Jpn J Pharmacol 87:61–66. doi:10.1254/jjp.87.61
Long J, Gao F, Tong L, Cotman CW, Ames BN, Liu J (2008) Mitochondrial decay in the brains of old rats: ameliorating effect of alpha-lipoic acid and acetyl-l-carnitine. Neurochem Res. doi:10.1007/s11064-008-9850-2
Navarro A, Boveris A (2004) Rat brain and liver mitochondria develop oxidative stress and lose enzymatic activities on aging. Am J Physiol Regul Integr Comp Physiol 287:R1244–R1249. doi:10.1152/ajpregu.00226.2004
Stephan K, Chang M, Brass EP, Hoppel CL (1991) Decreased activities of ubiquinol: ferricytochrome c oxidoreductase(complex and ferrocytochrome c:oxygen oxidoreductase (in liver mitochondria from rats with hydroxycobalamin-induced methylmalonic aciduria. J Biol Chem 266:20998–21003
Liu J, Yeo HC, Doniger SJ, Ames BN (1997) Assay of aldehydes from lipid peroxidation: gas chromatography-mass spectrometry compared to thiobarbituric acid. Anal Biochem 245:161–166. doi:10.1006/abio.1996.9990
Moreira PI, Santos MS, Moreno AM, Seica R, Oliveira CR (2003) Increased vulnerability of brain mitochondria in diabetic (Goto-Kakizaki) rats with aging and amyloid-beta exposure. Diabetes 52:1449–1456. doi:10.2337/diabetes.52.6.1449
Cossarizza A, Ceccarelli D, Masini A (1996) Functional heterogeneity of an isolated mitochondrial population revealed by cytofluorometric analysis at the single organelle level. Exp Cell Res 222:84–94. doi:10.1006/excr.1996.0011
Zamzami N, Metivier D, Kroemer G (2000) Quantitation of mitochondrial transmembrane potential in cells and in isolated mitochondria. Methods Enzymol 322:208–213. doi:10.1016/S0076-6879(00)22021-1
Hagen TM, Liu J, Lykkesfeldt J et al (2002) Feeding acetyl-l-carnitine and lipoic acid to old rats significantly improves metabolic function while decreasing oxidative stress. Proc Natl Acad Sci USA 99:1870–1875. doi:10.1073/pnas.261708898
Yang S, Tan TMC, Wee A, Leow CK (2004) Mitochondrial respiratory function and antioxidant capacity in normal and cirrhotic livers following partial hepatectomy. CMLS, Cell Mol Life Sci 61:220–229. doi:10.1007/s00018-003-3357-4
Janssen AJ, Trijbels FJ, Sengers RC et al (2007) Spectrophotometric assay for complex I of the respiratory chain in tissue samples and cultured fibroblasts. Clin Chem 53:729–734. doi:10.1373/clinchem.2006.078873
Zheng J, Ramirez VD (2000) Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Br J Pharmacol 130:1115–1123. doi:10.1038/sj.bjp.0703397
Hinman LM, Blass JP (1981) An NADH-linked spectrophotometric assay for pyruvate dehydrogenase complex in crude tissue homogenates. J Biol Chem 256:6583–6586
Keller JN, Mark RJ, Bruce AJ et al (1997) 4-Hydroxynonenal, an aldehydic product of membrane lipid peroxidation, impairs glutamate transport and mitochondrial function in synaptosomes. Neuroscience 80:685–696. doi:10.1016/S0306-4522(97)00065-1
Gould E, McEwen BS (1993) Neuronal birth and death. Curr Opin Neurobiol 3:676–682. doi:10.1016/0959-4388(93)90138-O
Long J, Wang X, Gao H et al (2007) d-Galactose toxicity in mice is associated with mitochondrial dysfunction: protecting effects of mitochondrial nutrient r-alpha-lipoic acid. Biogerontology 8:373–381. doi:10.1007/s10522-007-9081-y
Beppu M, Fukata Y, Kikugawa K (1988) Interaction of malondialdehyde-modified bovine serum-albumin and mouse peritoneal-macrophages. Chem Pharm Bull (Tokyo) 36:4519–4526
Buttkus H (1967) The reaction of myosin with malonaldehyde. J Food Sci 32:432–434. doi:10.1111/j.1365-2621.1967.tb09703.x
Ames BN, Elson SI, Silver EA (2002) High-dose vitamin therapy stimulates variant enzymes with decreased coenzyme binding affinity (increased K(m)): relevance to genetic disease and polymorphisms. Am J Clin Nutr 75:616–658
Ames BN, Liu J, Atamna H, Hagen TM (2003) Delaying the mitochondrial decay of aging in the brain. Clin Neurosci Res 2:331–338. doi:10.1016/S1566-2772(03)00010-0
Acknowledgments
The authors thank Dr. Edward Sharman for his critical reading and careful editing of this manuscript. This study was supported by an Outstanding Oversea Scholars Award from the Chinese Academy of Sciences, Shanghai Pujiang Talent Award, and a Hi-Sun Science and Technology Prize from Zhejiang Hi-Sun Pharmaceuticals, Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special issue article in honor of Dr. Akitane Mori.
Rights and permissions
About this article
Cite this article
Long, J., Liu, C., Sun, L. et al. Neuronal Mitochondrial Toxicity of Malondialdehyde: Inhibitory Effects on Respiratory Function and Enzyme Activities in Rat Brain Mitochondria. Neurochem Res 34, 786–794 (2009). https://doi.org/10.1007/s11064-008-9882-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-008-9882-7