Abstract
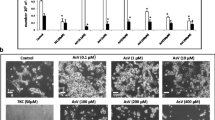
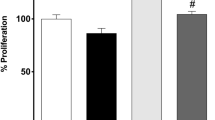
SH-SY5Y cells, derived from a human neuroblastoma, were submitted to short- or long-term exposures to lithium carbonate concentrations ranging from 0.5 to 8 mM. Short-term exposures (4 days) to concentrations higher than 6 mM were found to reduce cell growth rate while exposure to 8 mM resulted in significant cell mortality. These ranges of concentrations induced an overexpression of (1) the HSP27 stress protein, (2) a 108 kDa protein (P108) recognized by an anti-phospho-HSP27(Ser78) antibody, and probably corresponding to a phosphorylated HSP27 tetramer, (3) a 105 kDa protein (P105), possible glycosylated or phosphorylated form of the GRP94 stress protein and (4) a phosphorylated (inactivated) form of glycogen synthase kinase (GSK3α/β) SH-SY5Y cells, when cultured in the presence of 0.5 mM lithium for 25 weeks, displayed interesting features as compared to controls: (1) higher cell growth rate, (2) increased resistance toward the inhibitory effects of high lithium concentrations on cell proliferation, (3) lower basal level of lipid peroxidation (TBARS) and improved tolerance to oxidative stress induced by high lithium concentrations, (5) reduced expression of monomeric HSP27 versus an increase of corresponding tetrameric protein (P108) and (6) overexpression of a 105 kDa protein (P105). In conclusion, our study suggests that chronic treatment (over several months) by therapeutic relevant lithium concentrations could favour neurogenesis, decrease the vulnerability of neuronal cells to oxidative stress and induce posttranslational changes of molecular chaperones.







Similar content being viewed by others
References
Ikonomov OC, Manji HK (1999) Molecular mechanisms underlying mood stabilization in manic-depressive illness: the phenotype challenge. Am J Psychiatry 156:1506
Shaldubina A, Agam G, Belmaker RH (2001) The mechanism of lithium action: state of the art, ten years later. Prog Neuropsychopharmacol Biol Psychiatry 25:855–866. doi:10.1016/S0278-5846(01)00154-3
Bourin M, Prica C (2007) The role of mood stabilisers in the treatment of the depressive facet of bipolar disorders. Neurosci Biobehav Rev 31:963–975. doi:10.1016/j.neubiorev.2007.03.001
Noble W, Planel E, Zehr C, Olm V, Meyerson J, Suleman F et al (2005) Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc Natl Acad Sci USA 102:6990–6995. doi:10.1073/pnas.0500466102
Phiel CJ, Wilson CA, Lee VM, Klein PS (2003) GSK-3alpha regulates production of Alzheimer’s disease amyloid-beta peptides. Nature 423:435–439. doi:10.1038/nature01640
Paul C, Manero F, Gonin S, Kretz-Remy C, Virot S, Arrigo AP (2002) Hsp27 as a negative regulator of cytochrome c release. Mol Cell Biol 22:816–834
Engel T, Goñi-Oliver P, Lucas JJ, Avila J, Hernández F (2006) Chronic lithium administration to FTDP-17 tau and GSK-3beta overexpressing mice prevents tau hyperphosphorylation and neurofibrillary tangle formation, but pre-formed neurofibrillary tangles do not revert. J Neurochem 99:1445–1455. doi:10.1111/j.1471-4159.2006.04139.x
Cui J, Shao L, Young LT, Wang JF (2007) Role of glutathione in neuroprotective effects of mood stabilizing drugs lithium and valproate. Neuroscience 23:1447–1453. doi:10.1016/j.neuroscience.2006.11.010
Frey BN, Valvassori SS, Reus GZ, Martins MR, Petronilho FC, Bardini K, Quevedo J et al (2006) Effects of lithium and valproate on amphetamine-induced oxidative stress generation in an animal model of mania. J Psychiatry Neurosci 31:326–332
Shao L, Cui J, Young LT, Wang JF (2008) The effect of mood stabilizer lithium on expression and activity of glutathione s-transferase isoenzymes. Neuroscience 151:518–524. doi:10.1016/j.neuroscience.2007.10.041
Kitamura Y, Nomura Y (2003) Stress proteins and glial functions: possible therapeutic targets for neurodegenerative disorders. Pharmacol Ther 97:35–53. doi:10.1016/S0163-7258(02)00301-7
Smith RC, Rosen KM, Pola R, Magrané J (2005) Stress proteins in Alzheimer’s disease. Int J Hyperth 21:421–431. doi:10.1080/02656730500133165
Chuang DM (2005) The antiapoptotic actions of mood stabilizers: molecular mechanisms and therapeutic potentials. Ann NY Acad Sci 1053:195–204. doi:10.1196/annals.1344.018
Shao L, Sun X, Xu L, Young LT, Wang JF (2006) Mood stabilizing drug lithium increases expression of endoplasmic reticulum stress proteins in primary cultured rat cerebral cortical cells. Life Sci 78:1317–1323. doi:10.1016/j.lfs.2005.07.007
Renkawek K, Bosman GJ, de Jong WW (1994) Expression of small heat-shock protein hsp 27 in reactive gliosis in Alzheimer disease and other types of dementia. Acta Neuropathol 87:511–519
Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C et al (2000) Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol 2:645–652. doi:10.1038/35023595
Morimoto RI, Kroeger PE, Cotto JJ (1996) The transcriptional regulation of heat shock genes: a plethora of heat shock factors and regulatory conditions. In: Feige U, Moritimo I, Yahara I, Polla BS (eds) Stress-inducible cellular responses. Birkhauser-verlag, Basel, pp 139–163
Liu H, Lightfoot R, Stevens JL (1996) Activation of heat shock factor by alkylating agents is triggered by glutathione depletion and oxidation of protein thiols. J Biol Chem 271:4805–4812. doi:10.1074/jbc.271.9.4805
Croute F, Beau B, Murat JC, Vincent C, Komatsu H, Obata F et al (2005) Expression of stress-related genes in a cadmium-resistant A549 human cell-line. J Toxicol Environ Health A 68:703–718. doi:10.1080/15287390590925447
Pratt W (1998) The hsp90-based chaperone system: involvement in signal transduction from a variety of hormone and growth factor receptors. Proc Soc Exp Biol Med 17:420–434
Dickey CA, Kamal A, Lundgren K, Klosak N, Bailey RM, Dunmore J et al (2007) The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J Clin Invest 117:590–592. doi:10.1172/JCI29715
Lee AS (2001) The glucose-regulated proteins: stress induction and clinical applications. Biochem Sci 26:504–510. doi:10.1016/S0968-0004(01)01908-9
Ni M, Lee AS (2007) ER chaperones in mammalian development and human diseases. FEBS Lett 581:3641–3651. doi:10.1016/j.febslet.2007.04.045
Hoozemans JJ, Veerhuis R, Van Haastert ES, Rozemuller JM, Baas F, Eikelenboom P et al (2005) The unfolded protein response is activated in Alzheimer’s disease. Acta Neuropathol 110:165–172. doi:10.1007/s00401-005-1038-0
Yoo BC, Kim SH, Cairns N, Fountoulakis M, Lubec G (2001) Deranged expression of molecular chaperones in brains of patients with Alzheimer’s disease. Biochem Biophys Res Commun 280:249–258. doi:10.1006/bbrc.2000.4109
Hashimoto R, Senatorov V, Kanai H, Leeds P, Chuang DM (2003) Lithium stimulates progenitor proliferation in cultured brain neurons. Neurosciences 117(1):55–61
Wada A, Yokoo H, Yanagita T, Kobayashi H (2005) Lithium: potential therapeutics against acute brain injuries and chronic neurodegenerative diseases. J Pharmacol Sci 99:307–321. doi:10.1254/jphs.CRJ05009X
Plattner F, Angelo M, Giese KP (2006) The roles of cyclin-dependent kinase 5 and glycogen synthase kinase 3 in tau hyperphosphorylation. J Biol Chem 281:25457–25465. doi:10.1074/jbc.M603469200
Tandon DK, Dhawan , Nagpaul JP (1998) Effect of lithium on hepatic lipid peroxidation and antioxidative enzymes under different dietary protein regimens. J Appl Toxicol 18:187–190. doi :10.1002/(SICI)1099-1263(199805/06)18:3<187::AID-JAT495>3.0.CO;2-Y
Gould TD, Chen G, Manji HK (2004) In vivo evidence in the brain for lithium inhibition of glycogen synthase kinase-3. Neuropsychopharmacology 29:32–38. doi:10.1038/sj.npp. 1300283
Lowry OH, Rosebrouch NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Laemmli UK (1970) Cleavage of structural protein during the assembly of the head of bacteriophage T4. Nature 227:680–682. doi:10.1038/227680a0
Carbonneau MA, Peuchant E, Sess D, Canioni P, Clerc M (1991) Free and bound malondialdehyde measured as thiobarbituric acid adduct by HPLC in serum and plasma. Clin Chem 37:1423–1429
Hong YL, Yeh SL, Chang CY, Hu ML (2000) Total plasma malondialdehyde levels in 16 Taiwanese college students determined by various thiobarbituric acid tests and an improved high-performance liquid chromatography-based method. Clin Biochem 33:619–625. doi:10.1016/S0009-9120(00)00177-6
Schaffer B, Wiedau-Pazos M, Geschwind DH (2003) Gene structure and alternative splicing of glycogen synthase kinase-3 beta (GSK3β) in neural and non-neural tissues. Gene 302:73–81. doi:10.1016/S0378-1119(02)01092-2
Allagui MS, Vincent C, El Feki A, Gaubin Y, Croute C (2002) Lithium toxicity and expression of stress related-genes or -proteins in A549 cells. Biochim Biophys Acta 1773:1107–1115
Levine S, Saltzman A, Katof B, Meister A, Cooper TB (2002) Proliferation of glial cells induced by lithium in the neural lobe of the rat pituitary is enhanced by dehydration. Cell Prolif 35:167–172. doi:10.1046/j.1365-2184.2002.00235.x
Rao AS, Kremenevskaja N, Resch J, Brabant G (2005) Lithium stimulates proliferation in cultured thyrocytes by activating Wnt/beta-catenin signaling. Eur J Endocrinol 153:929–938. doi:10.1530/eje.1.02038
Misiuta IE, Saporta S, Sanberg PR, Zigova T, Willing AE (2006) Influence of retinoic acid and lithium on proliferation and dopaminergic potential of human NT2 cells. J Neurosci Res 835:668–679. doi:10.1002/jnr.20718
Korycka A, Robak T (1991) The effect of lithium on haematopoiesis of patients with acute myeloid leukaemia. Arch Immunol Ther Exp (Warsz) 39:501–509
Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HKJ (2000) Enhancement of hippocampal neurogenesis by lithium. Neurochem 75:1729–1734. doi:10.1046/j.1471-4159.2000.0751729.x
Kim JS, Chang MY, Yu IT, Kim JH, Lee SH, Lee YS et al (2004) Lithium selectively increases neuronal differentiation of hippocampal neural progenitor cells both in vitro and in vivo. J Neurochem 89:324–336. doi:10.1046/j.1471-4159.2004.02329.x
Manji HK, Moore JG, Chen G (1999) Lithium at 50: have the neuro-protective effects of this unique cation been overlooked. Biol Psychiatry 46:929–940. doi:10.1016/S0006-3223(99)00165-1
Nonaka S, Hough CJ, Chuang DM (1998) Chronic lithium treatment robustly protects neurons in the central nervous system against excitotoxicity by inhibiting N-methyl-D-aspartate receptor-mediated calcium influx. Proc Natl Acad Sci USA 95:2642–2647. doi:10.1073/pnas.95.5.2642
Chen RW, Chuang DM (1999) Long term lithium treatment suppresses p53 and Bax expression but increases Bcl-2 expression. J Biol Chem 274:6039–6042. doi:10.1074/jbc.274.10.6039
Brunello N (2004) Mood stabilizers: protecting the moodprotecting the brain. J Affect Disord 79:15–20. doi:10.1016/j.jad.2004.01.002
Zhou R, Gray NA, Yuan P, Li X, Chen J, Chen G et al (2005) The anti-apoptotic, glucocorticoid receptor cochaperone protein BAG-1 is a long-term target for the actions of mood stabilizers. Neurosci 25:4493–4502. doi:10.1523/JNEUROSCI.4530-04.2005
Ren M, Senatorov VV, Chen RW, Chuang DM (2003) Postinsult treatment with lithium reduces brain damage and facilitates neurological recovery in a rat ischemia/reperfusion model. Proc Natl Acad Sci USA 100:6210–6215. doi:10.1073/pnas.0937423100
Hiroi T, Wei H, Hough C, Leeds P, Chuang DM (2005) Protracted lithium treatment protects against the ER stress elicited by thapsigargin in rat PC12 cells: roles of intracellular calcium, GRP78 and Bcl-2. Pharmacogenomics J 5:102–111. doi:10.1038/sj.tpj.6500296
Cala SE (2000) GRP94 hyperglycosylation and phosphorylation in SF21 cells. Biochim Biophys Acta 1496:296–310. doi:10.1016/S0167-4889(00)00028-8
Zhang F, Phield CJ, Spece L, Gurvich N, Klein PS (2003) Inhibitory phosphorylation of glycogen synthase kinase-3 (GSK-3) in response to lithium. Evidence for autoregulation of GSK-3. J Biol Chem 278:33067–33077. doi:10.1074/jbc.M212635200
Mota de Freitas D, Castro MM, Geraldes CF (2006) Is competition between Li+ and Mg2+ underlying theme in the proposed mechanisms for the pharmacological action of lithium salts in bipolar disorder? Acc Chem Res 39:283–291. doi:10.1021/ar030197a
Sasaki T, Han F, Shioda N, Moriguchi S, Kasahara J, Ishiguro K et al (2006) Lithium-induced activation of Akt and CaM kinase II contributes to its neuroprotective action in a rat microsphere embolism model. Brain Res 1108:98–106. doi:10.1016/j.brainres.2006.06.009
Ehrnsperger M, Lilie H, Gaestel M, Buchner J (1999) The dynamics of Hsp25 quaternary structure. Structure and function of different oligomeric species. J Biol Chem 274:14867–14874. doi:10.1074/jbc.274.21.14867
Mehlen P, Kretz-Remy C, Preville X, Arrigo AP (1996) Human hsp27, Drosophila hsp27 and human alphaB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNFalpha-induced cell death. EMBO J 15:2695–2706
Arrigo AP, Virot S, Chaufour S, Firdaus W, Kretz-Remy C, Diaz-Latoud C (2005) Hsp27 consolidates intracellular redox homeostasis by upholding glutathione in its reduced form and by decreasing iron intracellular levels. Antioxid Redox Signal 7:414–422. doi:10.1089/ars.2005.7.414
Charette SJ, Lavoie JN, Lambert H, Landry J (2000) Inhibition of Daxx-mediated apoptosis by heat shock protein 27. Mol Cell Biol 20:7602–7612. doi:10.1128/MCB.20.20.7602-7612.2000
Mearow KM, Dodge ME, Rahimtula M, Yegappan C (2002) Stress-mediated signaling in PC12 cells––the role of the small heat shock protein, Hsp27, and Akt in protecting cells from heat stress and nerve growth factor withdrawal. J Neurochem 83:452–462. doi:10.1046/j.1471-4159.2002.01151.x
Wu R, Kausar H, Johnson P, Montoya-Durango DE, Merchant M, Rane MJ (2007) Hsp27 regulates Akt activation and polymorphonuclear leukocyte apoptosis by scaffolding MK2 to Akt signal complex. J Biol Chem 282:21598–21608. doi:10.1074/jbc.M611316200
Parcellier , Schmitt E, Gurbuxani S, Seigneurin-Berny D, Pance A, Chantome A, Plenchette S, Khochbin S, Solary E, Garrido C (2003) HSP27 is a ubiquitin-binding protein involved in I-kappaB alpha proteasomal degradation. Mol Cell Biol 23:5790–5802. doi:10.1128/MCB.23.16.5790-5802.2003
Lavoie JN, Lambert H, Hickey E, Weber LA, Landry J (1995) Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol Cell Biol 15:505–516
Mounier N, Arrigo AP (2002) Actin cytoskeleton and small heat shock proteins: how do they interact? Cell Stress Chaperones 7:167–176. doi :10.1379/1466-1268(2002)007<0167:ACASHS>2.0.CO;2
Rogalla T, Ehrnsperger M, Preville X, Kotlyarov A, Lutsch G, Ducasse C et al (1999) Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J Biol Chem 274:18947–18956. doi:10.1074/jbc.274.27.18947
Garrido C (2002) Size matters: of the small HSP27 and its large oligomers. Cell Death Differ 9:483–485. doi:10.1038/sj.cdd.4401005
Kosik KS, Shimura H (2005) Phosphorylated tau and the neurodegenerative foldopathies. Biochim Biophys Acta 1(739):298–310
Oktem F, Ozguner F, Sulak O, Olgar S, Akturk O, Yilmaz HR et al (2005) Lithium-induced renal toxicity in rats: protection by a novel antioxidant caffeic acid phenethyl ester. Mol Cell Biochem 277:109–115. doi:10.1007/s11010-005-5426-5
Shao L, Young T, Wang JF (2005) Chronic treatment with mood stabilizers lithium and valproate prevents excitotoxicity by inhibiting oxidative stress in rat cerebral cortical cells. Biol Psychiatry 58:879–884. doi:10.1016/j.biopsych.2005.04.052
Schafer M, Goodenough S, Moosmann B, Behl C (2004) Inhibition of glycogen synthase kinase 3 beta is involved in the resistance to oxidative stress in neuronal HT22 cells. Brain Res 1005:84–89. doi:10.1016/j.brainres.2004.01.037
King TD, Jope RS (2005) Inhibition of glycogen synthase kinase-3 protects cells from intrinsic but not extrinsic oxidative stress. NeuroReport 16:597–601. doi:10.1097/00001756-200504250-00016
Lai JS, Zhao C, Warsh JJ, lithium PP (2006) Cytoprotection by lithium and valproate varies between cell types and cellular stresses. Eur J Pharmacol 6:18–26. doi:10.1016/j.ejphar.2006.03.076
Acknowledgements
This work was supported by University Paul Sabatier (Toulouse, France) and Lions club Vallée du Girou, Toulouse.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Allagui, M.S., Nciri, R., Rouhaud, M.F. et al. Long-term Exposure to Low Lithium Concentrations Stimulates Proliferation, Modifies Stress Protein Expression Pattern and Enhances Resistance to Oxidative Stress in SH-SY5Y Cells. Neurochem Res 34, 453–462 (2009). https://doi.org/10.1007/s11064-008-9804-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-008-9804-8