Abstract
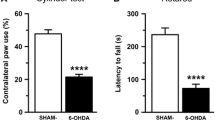
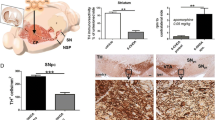
Recent evidence has linked striatal amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor function to the adverse effects of long-term dopaminergic treatment in Parkinson’s disease. The phosphorylation of AMPA subunit, GluR1, reflects AMPA receptor activity. To determine whether serine phosphorylation of GluR1 subunit by activation of Ca2+/calmodulin-dependent protein kinase II (CaMKII) contributes to the process, we examined the effects of unilateral nigrostriatal depletion with 6-hydroxydopamine and subsequent l-dopa treatment on motor responses and phosphorylation states. Three weeks of l-dopa administration to rats shortened the duration of the rotational response. We found a significant reduction in the abundance of both phosphorylated GluR1 at serine-831 site (pGluR1S831) and GluR1 in the cell plasma membrane of lesioned striatum. Chronic treatment of lesioned rats with l-dopa markedly upregulated the phosphorylation of GluR1 in lesioned striatum with a concomitant normalization of the plasma membrane GluR1 abundance, which lasted at least 1 day after withdrawal of chronic l-dopa treatment. Our immunostaining data showed that these changes were confined to parvalbumin-positive neurons where GluR1 subunits are exclusively expressed. Both the altered motor response duration and the degree of pGluR1S831 were attenuated by the intrastriatal administration of CaMKII inhibitor KN-93. These findings suggest that activation of CaMKII contributes to both development and maintenance of motor response duration alterations, through a mechanism that involves an increase in pGluR1S831 within parvalbumin-positive neurons.







Similar content being viewed by others
References
Standaert DG, Stern MB (1993) Update on the management of Parkinson’s disease. Med Clin N Am 77:169–183
Chase TN, Oh JD (2000) Striatal mechanisms and pathogenesis of Parkinsonian signs and motor complications. Ann Neurol 47:S122–S129
Obeso JA, Rodriguez-Oroz MC, Chana P, Lera G, Rodriguez M, Olanow CW (2000) The evolution and origin of motor complications in Parkinson’s disease. Neurology 55:S13–S20
Greenamyre JT (2000) New targets for therapy in Parkinson’s disease: pathogenesis and pathophysiology. Neurology 15:67–80
Nakanishi S, Nakajima Y, Masu M, Ueda Y, Nakahara K, Watanabe D, Yamaguchi S, Kawabata S, Okada M (1998) Glutamate receptors: brain function and signal transduction. Brain Res Rev 26:230–235
Konitsiotis S, Blanchet PJ, Verhagen L, Lamers E, Chase TN (2000) AMPA receptor blockade improves levodopa-induced dyskinesia in MPTP monkeys. Neurology 54:1589–1595
Marin C, Jimenez A, Bonastre M, Chase TN, Tolosa E (2000) Non-NMDA receptor-mediated mechanisms are involved in levodopa-induced motor response alterations in parkinsonian rats. Synapse 36:267–274
Papa SM, Chase TN (1996) Levodopa-induced dyskinesias improved by a glutamate antagonist in Parkinsonian monkeys. Ann Neurol 39:574–578
Oh JD, Chase TN (2002) Glutamate-mediated striatal dysregulation and the pathogenesis of motor response complications in Parkinson’s disease. Amino Acids 23:133–139
Barria A, Derkach V, Soderling T (1997) Identification of Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the amino-3-hydroxyl-5-methyl-4-isoxazole-propionate type gluatamate receptor. J Biol Chem 272:32727–32730
Mammen AL, Kameyame K, Roche KW, Huganir RL (1997) Phosphorylation of the amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem 272:32528–32533
Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL (1996) Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron 16:1179–1188
Banke TG, Bowie D, Lee H, Huganir RL, Schousboe A, Traynelis SF (2000) Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci 20:89–102
Benke TA, Luthi A, Isaac JT, Collingridge GL (1998) Modulation of AMPA receptor unitary conductance by synaptic activity. Nature 393:793–797
Brocke L, Chiang LW, Wagner PD, Schulman H (1999). Functional implications of the subunit composition of neuronal CaM kinase II. J Biol Chem 274:22713–22722
Sharp JW, Ross CM, Koehnle TJ, Gietzen DW (2004) Phosphorylation of Ca2+/calmodulin-dependent protein kinase type II and the alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (ampa) receptor in response to a threonine-devoid diet. Neuroscience 126:1053–1062
Oh JD, Vaughan CL, Chase TN (1999) Effect of dopamine denervation and dopamine agonist administration on serine phosphorylation of striatal NMDA receptor subunits. Brain Res 821:433–442
Tallaksen-Greene SJ, Wiley RG, Albin RL (1992) Localization of striatal excitatory amino acid binding site subtypes to striatonigral projection neurons. Brain Res 594:165–170
Tallaksen-Greene SJ, Albin RL (1994) Localization of AMPA-selective excitatory amino acid receptor subunits in identified populations of striatal neurons. Neuroscience 61:509–519
Chen Q, Veenman CL, Reiner A (1996) Cellular expression of ionotropic glutamate receptor subunits on specific striatal neuron types and its implication for striatal vulnerability in glutamate receptor-mediated excitotoxicity. Neuroscience 73:715–731
Wessell RH, Ahmed SM, Menniti FS, Dunbar GL, Chase TN, Oh JD (2004) NR2B selective NMDA receptor antagonist CP-101,606 prevents levodopa-induced motor response alterations in hemi-parkinsonian rats. Neuropharmacology 47:184–194
Brown AM, Deutch AY, Colbran RJ (2005) Dopamine depletion alters phosphorylation of striatal proteins in a model of Parkinsonism. Eur J Neurosci 22:247–256
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates, 4th edn. Academic Press
Papa SM, Engber TM, Kask AM, Chase TN (1994) Motor fluctuations in levodopa treated parkinsonian rats: relation to lesion extent and treatment duration. Brain Res 662:69–74
Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA (2002) Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. Eur J Neurosci 15:120–132
Anderson KD, Reiner A (1990) Extensive co-occurrence of substance P and dynorphin in striatal projection neurons: an evolutionarily conserved feature of basal ganglia organization. J Comp Neurol 295:339–369
Gerfen CR (1992) The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci 15:133–139
Reiner A, Anderson KD (1990) The patterns of neurotransmitter and neuropeptide co-occurrence among striatal projection neurons: conclusions based on recent findings. Brain Res Rev 15:251–265
DiFiglia M (1987) Synaptic organization of cholinergic neurons in the monkey neostriatum. J Comp Neurol 255:245–258
Cowan RL, Wilson CJ, Emson PC, Heizmann CW (1990) Parvalbumin-containing GABAergic interneurons in the rat neostriatum. J Comp Neurol 302:197–205
Figueredo-Cardenas G, Chen Q, Reiner A (1997) Age-dependent differences in survival of striatal somatostatin-NPY-NADPH-diaphorasecontaining interneurons versus striatal projection neurons after intrastriatal injection of quinolinic acid in rats. Exp Neurol 146:444–457
Dunah AW, Standaert DG (2001) Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane. J Neurosci 21: 5546–5558
Kwok KHH, Tse YC, Wong RNS, Yung KKL (1997) Cellular localization of GluR1, GluR2/3 and GluR4 glutamate receptor subunits in neurons of the rat neostriatum. Brain Res 778:43–55
Bernard V, Somogyi P, Bolam JP (1997) Cellular, subcellular, and subsynaptic distribution of AMPA-type glutamate receptor subunits in the neostriatum of the rat. J Neurosci 17: 819–833
Starr MS (1995) Glutamate/dopamine D1/D2 balance in the basal ganglia and its relevance to Parkinson’s disease. Synapse 19:264–293
Lange KW, Kornhuber J, Riederer P (1997) Dopamine/glutamate interactions in Parkinson’s disease. Neurosci Biobehav Rev 21:393–400
Perier C, Marin C, Bonastre M, Tolosa E, Hirsch EC (2002) AMPA receptor antagonist LY293558 reverses preproencephalin mRNA overexpression in the striatum of 6-OHDA-lesioned-rats treated with l-dopa. Eur J Neurosci 16:2236–2240
Papa SM, Boldry RC, Engber TM, Kask AM, Chase TN (1995) Reversal of levodopa-induced motor fluctuations in experimental parkinsonism by NMDA receptor blockade. Brain Res 701:13–18
Bolam JP, Hanley JJ, Booth PAC, Bevan MD (2000) Synaptic organisation of the basal ganglia. J Anat 196:527–542
Malinow R, Malenka RC (2002) AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25:103–126
Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, Wenthold RJ, Gallagher M, Huganir RL (2003) Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 112:631–643
Stefani A, Pisani A, Bernardi G, Bonci A, Mercuri NB, Stratta F, Calabresi P (1995) The modulation of dopamine receptors in rat striatum. J Neural Transm 45:61–66
Wickens JR, Kotter R, Alexander ME (1995) Effects of local connectivity on striatal function: stimulation and analysis of a model. Synapse 20:281–298
Hayashi Y, Ishida A, Katagiri H, Mishina M, Fujisawa H, Manabe T, Takahashi T (1997) Calcium- and calmodulin-dependent phosphorylation of AMPA type glutamate receptor subunits by endogenous protein kinases in the postsynaptic density. Brain Res Mol Brain Res 46:338–342
Acknowledgments
This study was supported by a project sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry and the Post-Project of Excellent Young Medical Person of Xihhua Hospital of Shanghai, China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Maowen Ba and Min Kong are contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ba, M., Kong, M., Yang, H. et al. Changes in Subcellular Distribution and Phosphorylation of GluR1 in Lesioned Striatum of 6-Hydroxydopamine-Lesioned and l-dopa-Treated Rats. Neurochem Res 31, 1337–1347 (2006). https://doi.org/10.1007/s11064-006-9177-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-006-9177-9