Abstract
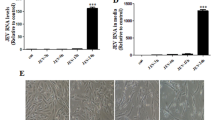
Increased expression of inducible nitric oxide synthase has been shown in murine Venezuelan equine encephalitis (VEE) virus infection. In this experimental model, melatonin (MTL) treatment has shown to be beneficial. The aim of this study was to determine the effect of VEE virus on the nitric oxide (NO) production and lipid peroxidation in neuroblastoma cell cultures, and to investigate the role of MTL during cell-virus interaction. Neuroblastoma cells were co-cultured with VEE virus and treated with MTL at doses ranging from 0 to 1.8 mM, for 6, 12, 24 and 48 h. NO and lipid peroxidation were measured in culture supernatants and in the cellular content by nitrite concentration and thiobarbituric acid assay, respectively. Expression of inducible nitric oxide synthase (iNOS) was determined by indirect immunofluorescence. Increased production of NO and lipid peroxidation products were found in supernatants and cellular contents of VEE virus treated cultures. Both NO and lipid peroxidation were decreased by MTL treatment in a time dependent manner. Increased iNOS expression was observed in VEE virus infected cultures that was reduced by MTL treatment. These results could be related to the beneficial role of MTL in the VEE experimental disease and address the possible therapeutic potential of the hormone in human VEE virus infection.





Similar content being viewed by others
References
Walton TE, Grayson MA (1988) Venezuelan equine encephalomyelitis. In: Monath T (ed), The arboviruses: epidemiology and ecology. CRC Press, Boca Raton, Florida pp 203–231
Weaver SC (1998) Recurrent emergence of Venezuelan equine encephalomyelitis. In: Scheld WM, Hughes J (eds), Emerging infection. ASM Press, Washington pp 27–42
Schoneboom BA, Catlin KM, Marty AM, Grieder FB (2000) Inflammation is a component of neurodegeneration in response to Venezuelan equine encephalitis virus infection in mice. J Neuroimmunol 109:132–146
Valero N, Larreal Y, Arias J, Espina LM, Maldonado M, Meleán E, Estévez J, Pérez M, Ávila J, Luzardo M, Moreli P, Urdaneta M (2004) Seroprevalencia de la encefalitis equina venezolana en una población de équidos del Estado Zulia, Venezuela, 1999–2001. Revista Científica XIV:324–330
Bonilla E, Valero-Fuenmayor N, Pons H, Bonilla-Chacín L (1997) Melatonin protects mice infected with Venezuelan Equine Encephalomyelitis Virus. Cell Mol Life Sci 53:430–434
Bonilla E, Rodón C, Valero N, Pons H, Chacín-Bonilla L, García J, Rodríguez Z, Medina-Leendertz S, Añez F (2001) Melatonin prolongs survival of immunodepressed mice infected with the Venezuelan equine encephalomyelitis virus. Trans Roy Soc Trop Med Hyg 95:207–210
Negrette B, Bonilla E, Valero N, Pons H, García J, Chacín-Bonilla L, Medina-Leendertz S, Añez F (2001) Melatonin treatment enhances the efficiency of mice immunization with Venezuelan equine encephalomyelitis virus TC-83. Neurochem Res 26:765–768
Valero N, Bonilla E, Pons H, Chacín-Bonilla L, Añez F, Espina LM, Medina-Leendertz S, García J (2002) Melatonin induces changes to serum cytokines in mice infected with the Venezuelan equine encephalomyelitis virus. Trans Roy Soc Trop Med Hyg 96:1–4
Bonilla E, Valero N, Chacín-Bonilla L, Pons H, Larreal Y, Medina-Leendertz S, Espina L (2003) Melatonin increases Interleukin-1( and decreases Tumor Necrosis Factor Alpha in the brain of mice infected with the Venezuelan Equine Encephalomyelitis Virus. Neurochem Res 28:687–692
Reiter RJ, Tan DX, Manchester LC, Qi W (2001) Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: a review of the evidence. Cell Biochem Biophys 34:237–256
Mannick JB (1995) The antiviral role of nitric oxide. Res Immunol 146:693–697
Schoneboom BA, Lee JS, Grieder FB (2000) Early expression of IFN-alpha/beta and iNOS in the brains of Venezuelan equine encephalitis virus-infected mice. Interferon Cytokine Res 20:205–215
Noda Y, Mori A, Liburdy R, Packer L (1999) Melatonin and its precursors scavenge nitric oxide. J Pineal Res 27:159–163
Archer S (1993) Measurement of nítric óxide in biological models. FASEB J 7:349–359
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite and N nitrate in biological fluids. Anal Biochem 126:131–138
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Horton JW (2003) Free radicals and lipid peroxidation mediated injury in burn trauma: the role of antioxidant therapy. Toxicology 189:75–88
Lopez-Farre A, Rodriguez-Feo JA, Sanchez de Miguel L, Rico L, Casado S (1998) Role of nitric oxide in the control of apoptosis in the microvasculature. J Biochem Cell Biol 30:1095–1106
Bonilla E, Valero N, Chacín-Bonilla L, Medina-Leendertz S (2004) Melatonin and viral infections. J Pineal Res 36:73–79
Chang HM, Tseng CY, Wei IH, Lue JH, Wen CY, Shieh JY (2005) Melatonin restores the cytochrome oxidase reactivity in the nodose ganglia of acute hypoxic rats. J Pineal Res 39:206–214
Kilic E, Kilic U, Reiter RJ, Bassetti CL, Hermann DM (2005) Tissue-plasminogen activator-induced ischemic brain injury is reversed by melatonin: role of iNOS and Akt. J Pineal Res 39:151–155
Lahiri DK, Ghosh C (1999) Interactions between melatonin, reactive oxygen species, and nitric oxide. Ann N Y Acad Sci 893:325–330
Mayo JC, Sainz RM, Antoli I, Herrera F, Martin V, Rodriguez C (2002) Melatonin regulation of antioxidant enzyme gene expression. Cell Mol Life Sci 59:1706–1713
Schuster C, Williams LM, Morris A, Morgan PJ, Barrett PT (2005) The human MT1 melatonin receptor stimulates cAMP production in the human neuroblastoma cell line SH-SY5Y cells via a calcium-calmodulin signal transduction pathway. J Neuroendocrinol 17:170–178
An Y, Liu E, Liu X, Yang F, Han F, Dai Q (2000) Protective effect of melatonin on neural cells against the cytotoxicity of oxyradicals. Chin Med Sci J 15:40–44
Obregon E, Punzon C, Fernandez-Cruz E, Fresno M, Munoz-Fernandez MA (1999) HIV-1 infection induces differentiation of immature neural cells through autocrine tumor necrosis factor and nitric oxide production. Virology 261:193–204
Chesler DA, Reiss CS (2002) IL-12, while beneficial, is not essential for the host response to VSV encephalitis. J Neuroimmunol 131:92–97
Chesler DA, McCutcheon JA, Reiss CS (2004) Posttranscriptional regulation of neuronal nitric oxide synthase expression by IFN-gamma. J Interferon Cytokine Res 24:141–149
Schoneboom BA, Fultz MJ, Miller TH, McKinney LC, Grieder FB (1999) Astrocytes as targets for Venezuelan equine encephalitis virus infection. J Neurovirol 5:342–354
Valero N, Espina LM, Añez G, Torres E, Mosquera J (2002) Short report: increased level of serum nitric oxide, in patients with dengue. Am J Trop Med Hyg 66:762–764
Ubol S, Hiriote W, Anuntagool N, Utaisincharoen PA (2001) Radical form of nitric oxide suppresses RNA synthesis of rabies virus. Virus Res 81:125–132
Neves-Souza PC, Azeredo EL, Zagne SM, Valk-De-Souza R, Reis SR, Cerqueira DI, Nogueira RM, Kubelka CF (2005) Inducible nitric oxide synthase (iNOS) is expressed in Dengue-infected patients during acute phase and monocytes infected in vitro. BMC Infect Dis 5:64
Rimmelzwaan G, Baars M, De Lister P, Fouchier R, Osterhaus A (1999) Inhibition of influenza virus replication by nitrin oxide. J Virol 73:8880–8883
Charnsilpa W, Takhampunya R, Endy TP, Mammen MP Jr, Libraty DH, Ubol S (2005) Nitric oxide radical suppresses replication of wild-type dengue 2 viruses in vitro. J Med Virol 77:89–95
Lin Y, Huang Y, Ma S, Yen C, Chiou S, Chen L, Liao C (1997) Inhibition of Japanese encephalitis virus infection by nitric oxide: antiviral effect of nitric oxide on RNA virus replication. J Virol 71:5227–5235
Palian BM, Reiss CS, Trottier MD (2005) VSV replication in neurons is inhibited by type I IFN at multiple stages of infection. Virology 333:215–225
Silvia OJ, Shellam GR, Urosevic N (2001) Innate resistance to flavivirus infection in mice controlled by Flv is nitric oxide-independent. J Gen Virol 82:603–607
Acknowledgments
This manuscript was supported by Consejo Cientifico y Humanistico de la Universidad del Zulia (CONDES) and FONACIT, Venezuela.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valero, N., Espina, L.M. & Mosquera, J. Melatonin Decreases Nitric oxide Production, Inducible Nitric oxide Synthase Expression and Lipid Peroxidation Induced by Venezuelan Encephalitis Equine Virus in Neuroblastoma Cell Cultures. Neurochem Res 31, 925–932 (2006). https://doi.org/10.1007/s11064-006-9098-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-006-9098-7
Keywords
- Venezuelan equine encephalitis
- Virus
- Togavirus
- Melatonin
- Nitric oxide
- Lipid peroxidation
- Neuroblastoma cell cultures
- Thiobarbituric acid
- Nitrites
- Virus infection
- Murine
- Neurons
- Protective
- In vivo
- Supernatants
- Cellular content
- In vitro
- Antioxidant activity
- Co-cultures
- Extracellular
- Malondialdehyde
- Nitric oxide synthase
- Central nervous system
- Inhibitory effect
- Viral replication
- Pathogenesis
- Brain