Abstract
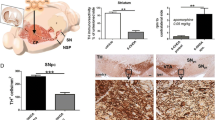
There is increasing evidence that, in addition to its function as the main neurotransmitter in the nigrostriatal pathway, dopamine (DA) may be neurotoxic in certain conditions. In this study, the toxicity of DA was assessed by direct injection into the substantia nigra of anaesthetised rats, and its effects were compared with those of 6-hydroxydopamine. Brains were removed 1, 2 and 3 weeks after the lesion for histological or neurochemical analysis. DA caused a significant loss of 35% of tyrosine hydroxylase-positive neurons in the pars compacta of substantia nigra and a 40% reduction of striatal DA content. Cells with signs compatible with both apoptosis and autophagy were observed. GADD153, a parameter of endoplasmic reticulum stress, was strongly induced by 6-hydroxydopamine but not by DA. DA increased the α-synuclein content 1 week after the lesion (but not at the later times analyzed) in tyrosine hydroxylase-positive and in non-dopaminergic fibers of pars reticulata. The α-synuclein increase may be a physiological temporal response to DA accumulation and/or to cell damage, but the simultaneous presence of α-synuclein and DA in the cell cytoplasm at concentration higher than normal is not exempt from risk. In fact, their incubation in a free cell system gives a stable dimerized form of α-synuclein that has been described as the critical rate-limiting step for its abnormal fibrillation.







Similar content being viewed by others
References
Barzilai A, Melamed E, Shirvan A (2001) Is there a rationale for neuroprotection against dopamine toxicity in Parkinson’s disease? Cell Mol Neurobiol 21:215–235
Barzilai A, Daily D, Zilkha-Falb R, Ziv I, Offen D, Melamed E, Shirvan A (2003) The molecular mechanisms of dopamine toxicity. Adv Neurol 91:73–82
Offen D, Ziv I, Panet H, Wasserman L, Stein R, Melamed E, Barzilai A (1997) Dopamine-induced apoptosis is inhibited in PC12 expressing bcl-2. Cell Mol Neurobiol 17:289–304
Lai C-T, Yu PH (1997) Dopamine- and l-ß-3,4-dihydroxyphenylalanine hydrochloride (l-dopa)-induced cytotoxicity towards catecholaminergic neuroblastoma SH-SY5Y cells. Biochem Pharmacol 53:363–372
Michel PP, Hefti F (1990) Toxicity of 6-hydroxydopamine and dopamine for dopaminergic neurons in culture. J Neurosci Res 25:428–435
McLaughlin BA, Nelson D, Erecinska M, Chesselet MF (1998) Toxicity of dopamine to striatal neurons in vitro and potentiation of cell death by mitochondrial inhibitors. J Neurochem 70:2406–2415
Filloux F, Townsend J (1993) Pre- and post-synaptic neurotoxic effects of dopamine demonstrated by intrastriatal injection. Exp Neurol 119:79–88
Hattori A, Luo Y, Umegaki H, Munoz J, Roth GS (1998) Intrastriatal injection of dopamine results in DNA damage and apoptosis in rats. Neuroreport 9:2569–2572
Ungerstedt U (1976) 6-Hydroxydopamine-induced degeneration of the nigrostriatal dopamine pathway: the turning syndrome. Pharmacol Ther 2:37–40
Slivka A, Cohen G (1985) Hydroxyl radical attack on dopamine. J Biol Chem 260:15466–15472
Sulzer D, Bogulavsky J, Larsen KE, Behr GE, Karatekin E, Kleinman MH, Turro N, Krantz D, Edwards RH, Greene LA, Zecca L (2000) Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc Natl Acad Sci USA 97:11869–11874
Simantov R, Blinder E, Ratovitski T, Tauber M, Gabbay M, Porat S (1996) Dopamine-induced apoptosis in human neuronal cell: inhibition by nucleic acids antisense to the dopamine transported. Neuroscience 74:39–50
Gómez-Santos C, Ferrer I, Santidrián AF, Barrachina M, Gil J, Ambrosio S (2003) Dopamine induces autophagic cell death and α-synuclein increase in human neuroblastoma SH-SY5Y cells. J Neurosci Res 73:341–350
Anglade P, Vyas S, Javoy-Agid F, Herrero MT, Michel PP, Márquez J, Mouatt-Prigent A, Ruberg M, Hirsch EC, Agid Y (1997) Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histol Histopathol 12:25–31
Stokes AH, Freeman WM, Mitchell SG, Burnette TA, Hellmann GM, Vrana KE (2002) Induction of GADD45 and GADD153 in neuroblastoma cells by dopamine-induced toxicity. Neurotoxicol 23:675–684
Holtz WA, O’Malley KL (2003) Parkinsonian mimetics induce aspects of unfolded protein response in death of dopaminergic neurons. J Biol Chem 278:19367–19377
Oyadomari S, Mori M (2004) Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11:381–389
Gómez-Santos C, Barrachina M, Giménez-Xavier P, Dalfó E, Ferrer I, Ambrosio S (2005) Induction of C/EBPß and GADD153 expression by dopamine in human neuroblastoma cells; relationship with α-synuclein increase and cell damage. Brain Res Bull 65:87–95
Bennett MC (2005) The role of α-synuclein in neurodegenerative diseases. Pharmacol Ther 103:311–331
Vila M, Przedborski S (2004) Genetic clues to the pathogenesis of Parkinson’s disease. Nature Med 10 Suppl:S58–S62
Espino A, Llorens J, Calopa M, Bartrons R, Rodriguez-Farré E, Ambrosio S (1995) Cerebrospinal dopamine metabolites in rats after intrastriatal administration of 6-hydroxydopamine or 1-methyl-4-phenylpyridinium ion. Brain Res 669:19–25
Kearns CM, Gash DM (1995) GDNF protects nigral dopamine neurons against 6-hydroxydopamine in vivo. Brain Res 672:104–111
Schmued LC, Albertson C, Slikker A Jr (1997) Fluoro-jade: a novel fluorochrome for the sensitive reliable histochemical localization of neuronal degeneration. Brain Res 751:37–46
Aroca P, Solano F, García-Borrón JC, Lozano JA (1990) A new spectrophotometric assay for dopachrome tautomerase. J Biochem Biophys Methods 21:35–46
Ariano MA, Grissell AE, Littlejohn FC, Buchanan TM, Elsworth JD, Collier TJ, Steece-Collier K (2005) Partial dopamine loss enhances activated caspase-3 activity: differential outcomes in striatal projection systems. J Neurosci Res 82:387–396
Altar CA, Marien M, Marshall JF (1987) Time course of adaptations in dopamine biosynthesis, metabolism, and release following nigrostriatal lesions: implications for behavioral recovery from brain injury. J Neurochem 48:390–399
Zuch CL, Nordstroem VK, Briedrick LA, Hoering GR, Granholm AC, Bickford PC (2000) Time course of degenerative alterations in nigral dopaminergic neurons following a 6-hydroxydopamine lesion. J Comp Neurol 427: 440–454
Kopin IJ (1993) Neurotransmitters and disorders of the basal ganglia. In: Siegel GJ, Agranoff BW, Albers RW, Molinoff PB (eds). Basic Neurochemistry. Raven Press, New York, p 93
Baker AJ, Zornow MH, Scheller MS, Yaksh TL, Skilling SR, Smullin DH, Larson AA, Kuczenski R (1991) Changes in extracellular concentrations of glutamate, aspartate, glycine, dopamine, serotonin, and dopamine metabolites after transient global ischemia in the rabbit brain. J Neurochem 57:1370–1379
Gómez C, Ferrer I, Reiriz J, Viñals F, Barrachina M, Ambrosio S (2001) Low concentrations of 1-methyl-4-phenylpyridinium ion induce caspase-mediated apoptosis in human SH-SY5Y neuroblastoma cells. Brain Res 935:32–39
Liang Q, Liou AK, Ding Y, Cao G, Xiao X, Perez RG, Chen J (2004) 6-Hydroxydopamine induces dopaminergic cell degeneration caspase-9-mediated apoptotic pathway that is attenuated by caspase-9dn expression. J Neurosci Res 77: 747–761
Junn E, Mouradian MM (2001) Apoptotic signaling in dopamine-induced cell death: the role of oxidative stress, p38 mitogen-activated protein kinase, cytochrome c and caspases. J Neurochem 78:374–383
Cutillas B, Espejo M, Gil J., Ferrer I, Ambrosio S (1999) Caspase inhibition protects nigral neurons against 6-hydroxydopamine-induced retrograde degeneration. Neuroreport 10:2605–2608
Conn KJ, Gao W-W, Ullman MD, McKeon-O’Malley C, Eisenhauer PB, Fine RE, Wells JM (2002) Specific up-regulation of GADD153/CHOP in 1-methyl-4-phenyl-pyridinium-treated SH-SY5Y cells. J Neurosci Res 68:755–760
Giménez-Xavier P, Gómez-Santos C, Castaño E, Francisco R, Boada J, Unzeta M, Sanz E, Ambrosio S (2006) The decrease of NAD(P)H has a prominent role in dopamine toxicity. Biochim Biophys Acta 1762:564–574
Harrington KA, Augood SJ, Kingsbury AE, Foster OJF, Emson PC (1996) Dopamine transported (DAT) and synaptic vesicle amine transporter (VMAT2) gene expression in the substantia nigra of control and Parkinson’s disease. Mol Brain Res 36:157–162
Fahn S (1997) Levodopa-induced neurotoxicity, does it represent a problem for the treatment of Parkinson’s disease? CNS Drugs 8:376–393
Bustamante D, You Z-B, Castel M-N, Johansson S, Goiny M, Terenius L, Hökfelt T, Herrera-Marschitz M (2002) Effect of single and repeated methamphetamine treatment on neurotransmitter release in substantia nigra and neostriatum of the rat. J Neurochem 83:645–654
Sidhu A, Wersinger C, Moussa CEH, Vernier P (2004) The role of α-synuclein in both neuroprotection and neurodegeneration. Ann N Y Acad Sci 1035:250–270
Vila M, Vukasovic S, Jackson LV, Neystat M, Jakowec M, Przedborski S (2000) α-Synuclein up-regulation in substantia nigra of dopaminergic neurons following administration of the parkinsonian toxin MPTP. J Neurochem 74:721–729
Conway KA, Rochet JC, Bieganski RM, Landsbury PT (2001) Kinetic stabilization of the α-synuclein protofibril by a dopamine-α-synuclein adduct. Science 294:1346–1349
Souza JM, Giasson BI, Chen Q, Lee VM Y, Ischiropoulos H (1999) Dityrosine cross-linking promotes formation of stable α-synuclein polymers. J Biol Chem 275:18344–18349
Krishnan S, Chi EY, Wood SJ, Kendrick BS, Li C, Garzón-Rodríguez W, Wypych J, Randolph TW, Narhi L-O, Biere AL, Citron M, Carpenter JF (2003) Oxidative dimer formation is the critical rate-limiting step for Parkinson’s disease α-synuclein fibrillogenesis. Biochemistry 42:829–837
Linert W, Jameson GNL (2000) Redox reactions of neurotransmitters possibly involved in the progression of Parkinson’s disease. J Inorg Biochem 79:319–326
Norris EH, Giasson BI, Hodara R, Xu S, Trojanowski JQ, Ischiropoulos H, Lee VM (2005) Reversible inhibition of alpha-synuclein fibrillization by dopaminochrome-mediated conformational alterations. J Biol Chem 280:21212–21219
Acknowledgment
We thank the members of the Biochemistry, Neuropathology and Biology Unit (Serveis Científic-Tècnics of Campus Bellvitge) of the University of Barcelona. We are also grateful to Robin Rycroft for help with the English. This study was supported by the BFI 2003-02883, FIS 02/0004 and FIS-CIEN C-03006 grants from the Spanish Government and from the Fundació La Marató-TV3 (010310).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gómez-Santos, C., Giménez-Xavier, P., Ferrer, I. et al. Intranigral Dopamine Toxicity and α-Synuclein Response in Rats. Neurochem Res 31, 861–868 (2006). https://doi.org/10.1007/s11064-006-9090-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-006-9090-2