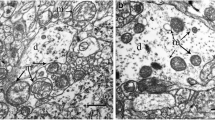
Brain ischemia results in neuronal injury and neurological disability. The present study examined the effect of mild (6% O2) and severe (2% O2) hypoxia on mitochondria of rat cortical synaptosomes. During mild and severe hypoxia, JO2 and ATP production significantly decreased and mitochondrial membranes depolarized. Synaptosomal calcium concentration increased slightly, albeit not significantly. After a 1 h re-oxygenation period, JO2, ATP production and mitochondrial membrane potential returned to control levels in synaptosomes incubated in 6% O2. In synaptosomes incubated in 2% O2, however, the ATP production was not restored after re-oxygenation and intrasynaptosomal Ca2+ significantly increased. The results indicate that both mild and severe hypoxia influence the physiology of synaptosomal mitochondria; the modifications are reversible after mild hypoxia and but partly irreversible after severe hypoxia.
Similar content being viewed by others
References
J. I. Jeong Y. W. Lee Y. K. Kim (2003) ArticleTitleChemical hypoxia-induced cell death in human glioma cells: role of reactive oxygen species, ATP depletion, mitochondrial damage and Ca2+ Neurochem. Res. 28 1201–1211 Occurrence Handle10.1023/A:1024280429036 Occurrence Handle12834260
D. R. Green G. Kroemer (2004) ArticleTitleThe pathophysiology of mitochondrial cell death Science 305 626–629 Occurrence Handle10.1126/science.1099320 Occurrence Handle15286356
G. Kroemer J. C. Reed (2000) ArticleTitleMitochondrial control of cell death Nat. Med. 6 513–519 Occurrence Handle10.1038/74994 Occurrence Handle10802706
S. Desagher J.-C Martinou (2004) ArticleTitleMitochondria as the central control point of apoptosis Trends Cell Biol. 10 369–377 Occurrence Handle10.1016/S0962-8924(00)01803-1
O. P. Mishra M. Delivoria-Papadopoulos (2004) ArticleTitleInositol tetrakisphosphate (IP4)- and inositol triphosphate (IP3)-dependent Ca2+ influx in cortical neuronal nuclei of newborn piglets following graded hypoxia Neurochem. Res. 29 391–396 Occurrence Handle10.1023/B:NERE.0000013742.19074.7e Occurrence Handle15002735
D. Mottet G. Michel P. Renard N. Ninane M. Raes C. Michiels (2002) ArticleTitleRole of ERK and calcium in the hypoxia-induced activation of HIF−1 J. Cell Physiol. 194 30–44 Occurrence Handle10.1002/jcp.10176
A. Andreyev G. Fiskum (1999) ArticleTitleCalcium induced release of mitochondrial cytochrome c by different mechanisms selective for brain versus liver Cell Death Differ. 6 825–832 Occurrence Handle10.1038/sj.cdd.4400565 Occurrence Handle10510464
L. Bambrick T. Kristian G. Fiskum (2004) ArticleTitleAstrocyte mitochondrial mechanisms of ischemic brain injury and neuroprotection Neurochem. Res. 29 601–608 Occurrence Handle10.1023/B:NERE.0000014830.06376.e6 Occurrence Handle15038607
T. Kristian J. Gertsch T. E. Bates B. K. Siesjo (2000) ArticleTitleCharacteristics of the calcium-triggered mitochondrial permeability transition in nonsynaptic brain mitochondria: effect of cyclosporin A and ubiquinone O J. Neurochem. 74 1999–2009 Occurrence Handle10.1046/j.1471-4159.2000.0741999.x Occurrence Handle10800943
M. A. Perez-Pinzon T. J. Sick M. Rosenthal (1999) ArticleTitleMechanism(s) of mitochondrial hyperoxidation after global cerebral ischemia Adv. Exp. Med. Biol. 471 175–180 Occurrence Handle10659145
Lai J. F. K., and Clark J. B. 1989. Isolation and characterization of synaptic and non-synaptic mitochondria from mammalian brain. Pages 43–97. in Boulton A. B. and Barker G. B. (eds), Humana Press
A. G. Gornall C. J. Bardawil M. M. David (1949) ArticleTitleDetermination of serum protein by means of the biuret reaction J. Biol. Chem. 177 751–766
B. Reynafarje L. E. Costa A. L. Lehninger (1985) ArticleTitleO2 solubility in aqueous media determined by a kinetic method Anal. Biochem. 145 406–418 Occurrence Handle10.1016/0003-2697(85)90381-1 Occurrence Handle4014672
T. R. Hesketh G. A. Smith J. P. Moore M. V. Taylor J. C. Metcalfe (1983) ArticleTitleFree cytoplasmic calcium concentration and the mitogenic stimulation of lymphocytes J. Biol. Chem. 258 4876–4882 Occurrence Handle6601105
R. Y. Tsien T. Pozzan T. J. Rink (1982) ArticleTitleCalcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator J. Cell Biol. 94 325–338 Occurrence Handle10.1083/jcb.94.2.325 Occurrence Handle6980885
G. Grynkiewicz M. Poenie R. Y. Tsien (1985) ArticleTitleA new generation of Ca2+ indicators with greatly improved fluorescence properties J. Biol. Chem. 260 3440–3450 Occurrence Handle3838314
K. J. Banasiak Y. Xia G. G. Haddad (2000) ArticleTitleMechanisms underlying hypoxia-induced neuronal apoptosis Prog. Neurobiol. 62 215–249 Occurrence Handle10.1016/S0301-0082(00)00011-3 Occurrence Handle10840148
A. M. Gorman S. Ceccatelli S. Orrenius (2000) ArticleTitleRole of mitochondria in neuronal apoptosis Dev. Neurosci. 22 348–358 Occurrence Handle10.1159/000017460 Occurrence Handle11111150
K. L. Allen A. Almeida T. E. Bates J. B. Clark (1995) ArticleTitleChanges of respiratory chain activity in mitochondrial and synaptosomal fractions isolated from the gerbil brain after graded ischaemia J. Neurochem. 64 2222–2229 Occurrence Handle7722507
S. L. Budd Haeberlein (2004) ArticleTitleMitochondrial function in apoptotic neuronal cell death Neurochem. Res. 29 521–530 Occurrence Handle10.1023/B:NERE.0000014823.74782.b7 Occurrence Handle15038600
R. J. Miller (1991) ArticleTitleThe control of neuronal Ca2+ homeostasis Prog. Neurobiol. 37 255–285 Occurrence Handle10.1016/0301-0082(91)90028-Y Occurrence Handle1947178
J. Keelan T. E. Bates J. B. Clark (1996) ArticleTitleIntrasynaptosomal free calcium concentration during rat brain development: effects of hypoxia, aglycaemia, and ischaemia J. Neurochem. 66 2460–2467 Occurrence Handle8632170
J. W. Lazarewicz M. O. Samoilov D. G. Semenov (1987) ArticleTitleChanges of intracellular calcium homeostasis in brain cortical structures during anoxia in vivo and in vitro Resuscitation 15 245–255 Occurrence Handle10.1016/0300-9572(87)90003-7 Occurrence Handle2831597
F. Dagani R. Ferrari I. Canevari (1990) ArticleTitleA pharmacological model for studying the role of NA+ gradients in the modulation of synaptosomal free [Ca2+]i levels and energy metabolism Brain Res. 530 261–266 Occurrence Handle10.1016/0006-8993(90)91293-P Occurrence Handle2176115
F. Dagani R. Ferrari P. Tosca L. Canevari (1992) ArticleTitleEffects of calcium antagonists on glycolysis of rat brain synaptosomes Biochem. Pharmacol. 43 371–374 Occurrence Handle10.1016/0006-2952(92)90300-8 Occurrence Handle1531411
S. Kokura N. Yoshida T. Yoshikawa (2002) ArticleTitleAnoxia/reoxygenation-induced leukocyte-endothelial cell interactions Free Rad. Biol. Med. 33 427–432 Occurrence Handle10.1016/S0891-5849(02)00852-3 Occurrence Handle12160924
A. Meini A. Benocci M. Frosini G. P. Sgaragli J. Blanco Garcia G. P. Pessina C. Aldinucci M. Palmi (2003) ArticleTitlePotentiation of intracellular Ca2+ mobilization by hypoxia-induced NO generation in rat brain striatal slices and human astrocytoma U−373 MG cells and its involvement in tissue damage Eur. J. Neurosci. 17 692–700
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aldinucci, C., Carretta, A. & Pessina, G. The Effect of Mild and Severe Hypoxia on Rat Cortical Synaptosomes. Neurochem Res 30, 981–987 (2005). https://doi.org/10.1007/s11064-005-6529-9
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11064-005-6529-9