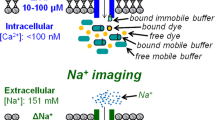
The estimation of not only concentrations of different intracellular ions (calcium in particular), but also of the dynamics of changes in these parameters, is one of the most important tasks in today cell biology. The measurements of calcium concentrations in the cell and even in its separate organelles are possible with the use of several experimental approaches (electron microscopy, electrophysiological techniques, fluorescent/optic methods, and others). Calcium is present in the cell in free (ionized) and bound states. Local rapid changes in the Ca2+ level in definite cell sites are individual quanta of an integral oscillatory calcium signal determining numerous cell functions. Separation of calcium fluxes in different cell compartments and evaluation of the role of calcium receptors and channels in the plasma membrane and membranes of the intracellular organelles allows experimenters to begin estimation of contributions of the respective events to the regulation of physiological functions of the cell, e.g., of synaptic plasticity of the neuron. This review describes some methodic approaches for the measurements of concentrations of calcium and characteristics of its fluxes; this makes it possible to characterize separate components of calcium signaling and to determine the roles of these components in the regulation of different functions of excitable cells.
Similar content being viewed by others
References
M. J. Berridge, “Neuronal calcium signaling,” Neuron, 21, 13-26 (1998).
T. Pozzan, R. Rizzuto, P. Volpe, and J. Meldolesi, “Molecular and cellular physiology of intracellular calcium stores,” Physiol. Rev., 74, 595-636 (1994).
M. J. Berridge, P. Lipp, and M. D. Bootman, “The versatility and universality of calcium signalling,” Nat. Rev. Mol. Cell Biol., 1, 11-21 (2000).
D. Futagi, and K. Kitano, “Ryanodine-receptor-driven intracellular calcium dynamics underlying spatial association of synaptic plasticity,” J. Comput. Neurosci., 39, 329-347 (2015).
A. V. Somlyo, H. Shuman, and A. P. Somlyo, “Composition of sarcoplasmic reticulum in situ by electron probe X-ray microanalysis,” Nature, 268, 556-558 (1977).
T. A. Hall, and B. L. Gupta, “The localization and assay of chemical elements by microprobe methods,” Q. Rev. Biophys., 16, 279-339 (1983).
S. B. Andrews, R. A. Buchanan, and R. D. Leapman, “Quantitative dark-field mass analysis of ultrathin cryosections in the field-emission scanning transmission electron microscope,” Scanning Microsc. Suppl., 8, 13-23; discussion 23-14 (1994).
R. A. Buchanan, R. D. Leapman, M. F. O’Connell, et al., “Quantitative scanning transmission electron microscopy of ultrathin cryosections: subcellular organelles in rapidly frozen liver and cerebellar cortex,” J. Struct. Biol., 110, 244-255 (1993).
R. D. Leapman, and S. B. Andrews, “Analysis of directly frozen macromolecules and tissues in the field-emission STEM,” J. Microsc., 161, 3-19 (1991).
L. D. Pozzo-Miller, N. B. Pivovarova, J. A. Connor, et al., “Correlated measurements of free and total intracellular calcium concentration in central nervous system neurons,” Microsc. Res. Tech., 46, 370-379 (1999).
E. Neher, “The use of fura-2 for estimating Ca buffers and Ca fluxes,” Neuropharmacology, 34, 1423-1442 (1995).
R. D. Leapman, S. Q. Sun, J. A. Hunt, and S. B. Andrews, “Biological electron energy loss spectroscopy in the field-emission scanning transmission electron microscope,” Scanning Microsc. Suppl., 8, 245-258; discussion 258-249 (1994).
H. Shuman, and A. P. Somlyo, “Electron energy loss analysis of near-trace-element concentrations of calcium,” Ultramicroscopy, 21, 23-32 (1987).
A. B. Borle, “An overview of techniques for the measurement of calcium distribution, calcium fluxes, and cytosolic free calcium in mammalian cells,” Environ. Health Perspect., 84, 45-56 (1990).
T. Uchikawa, and A. B. Borle, “Studies of calcium-45 desaturation from kidney slices in flow-through chambers,” Am. J. Physiol., 234, R34-38 (1978).
A. Takahashi, P. Camacho, J. D. Lechleiter, and B. Herman, “Measurement of intracellular calcium,” Physiol. Rev., 79, 1089-1125 (1999).
S. Baudet, L. Hove-Madsen, and D. M. Bers, “How to make and use calcium-specific mini- and microelectrodes,” Methods Cell Biol., 40, 93-113 (1994).
S. Q. Wang, L. S. Song, E. G. Lakatta, and H. Cheng, “Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells,” Nature, 410, 592-596 (2001).
B. L. Sabatini, and W. G. Regehr, “Optical measurement of presynaptic calcium currents,” Biophys. J., 74, 1549-1563 (1998).
S. M. Baylor, W. K. Chandler, and M. W. Marshall, “Calcium release and sarcoplasmic reticulum membrane potential in frog skeletal muscle fibres,” J. Physiol., 348, 209-238 (1984).
G. Brum, E. Rios, and E. Stefani, “Effects of extracellular calcium on calcium movements of excitation-contraction coupling in frog skeletal muscle fibres,” J. Physiol., 398, 441-473 (1988).
O. Delbono, and G. Meissner, “Sarcoplasmic reticulum Ca2+ release in rat slow- and fast-twitch muscles,” J. Membr. Biol., 151, 123-130 (1996).
J. Garcia, and M. F. Schneider, “Calcium transients and calcium release in rat fast-twitch skeletal muscle fibres,” J. Physiol., 463, 709-728 (1993).
W. Melzer, E. Rios, and M.F. Schneider, “A general procedure for determining the rate of calcium release from the sarcoplasmic reticulum in skeletal muscle fibers,” Biophys. J., 51, 849-863 (1987).
N. Shirokova, J. Garcia, G. Pizarro, and E. Rios, “Ca2+ release from the sarcoplasmic reticulum compared in amphibian and mammalian skeletal muscle,” J. Gen. Physiol., 107, 1-18 (1996).
S. M. Baylor, W. K. Chandler, and M. W. Marshall, “Sarcoplasmic reticulum calcium release in frog skeletal muscle fibres estimated from Arsenazo III calcium transients,” J. Physiol., 344, 625-666 (1983).
W. Melzer, E. Rios, and M. F. Schneider, “Time course of calcium release and removal in skeletal muscle fibers,” Biophys. J., 45, 637-641 (1984).
E. Rios, M. D. Stern, A. Gonzalez, et al., “Calcium release flux underlying Ca2+ sparks of frog skeletal muscle,” J. Gen. Physiol., 114, 31-48 (1999).
L. Figueroa, V. M. Shkryl, J. Zhou, et al., “Synthetic localized calcium transients directly probe signalling mechanisms in skeletal muscle,” J. Physiol., 590, 1389-1411 (2012).
H. Cheng, W. J. Lederer, and M. B. Cannell, “Calcium sparks: elementary events underlying excitationcontraction coupling in heart muscle,” Science, 262, 740-744 (1993).
A. Tsugorka, E. Rios, and L.A. Blatter, “Imaging elementary events of calcium release in skeletal muscle cells,” Science, 269, 1723-1726 (1995).
I. Parker, and I. Ivorra, “Localized all-or-none calcium liberation by inositol trisphosphate,” Science, 250, 977-979 (1990).
D. J. Santiago, J. W. Curran, D. M. Bers, et al., “Ca sparks do not explain all ryanodine receptor-mediated SR Ca leak in mouse ventricular myocytes,” Biophys. J., 98, 2111-2120 (2010).
V. M. Shkryl, L. A. Blatter, and E. Rios, “Properties of Ca2+ sparks revealed by four-dimensional confocal imaging of cardiac muscle,” J. Gen. Physiol., 139, 189-207 (2012).
C. H. Kong, D. R. Laver, and M. B. Cannell, “Extraction of sub-microscopic Ca fluxes from blurred and noisy fluorescent indicator images with a detailed model fitting approach,” PLoS Comput. Biol., 9, e1002931 (2013).
C. Kettlun, A. Gonzalez, E. Rios, and M. Fill, “Unitary Ca2+ current through mammalian cardiac and amphibian skeletal muscle ryanodine receptor channels under nearphysiological ionic conditions,” J. Gen. Physiol., 122, 407-417 (2003).
M. E. Larkum, S. Watanabe, T. Nakamura, et al., “Synaptically activated Ca2+ waves in layer 2/3 and layer 5 rat neocortical pyramidal neurons,” J. Physiol., 549, 471-488 (2003).
L. D. Pozzo Miller, J. J. Petrozzino, G. Golarai, and J. A. Connor, “Ca2+ release from intracellular stores induced by afferent stimulation of CA3 pyramidal neurons in hippocampal slices,” J. Neurophysiol., 76, 554-562 (1996).
A. Verkhratsky, “Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons,” Physiol. Rev., 85, 201-279 (2005).
I. Llano, J. Gonzalez, C. Caputo, et al., “Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients,” Nat. Neurosci., 3, 1256-1265 (2000).
C. Lohmann, A. Finski, and T. Bonhoeffer, “Local calcium transients regulate the spontaneous motility of dendritic filopodia,” Nat. Neurosci., 8, 305-312 (2005).
S. Manita, and W. N. Ross, “Synaptic activation and membrane potential changes modulate the frequency of spontaneous elementary Ca2+ release events in the dendrites of pyramidal neurons,” J. Neurosci., 29, 7833-7845 (2009).
R. Rizzuto, and T. Pozzan, “Microdomains of intracellular Ca2+: molecular determinants and functional consequences,” Physiol. Rev., 86, 369-408 (2006).
C. M. Niswender, and P. J. Conn, “Metabotropic glutamate receptors: physiology, pharmacology, and disease,” Annu. Rev. Pharmacol. Toxicol., 50, 295-322 (2010).
M. Kano, O. Garaschuk, A. Verkhratsky, and A. Konnerth, “Ryanodine receptor-mediated intracellular calcium release in rat cerebellar Purkinje neurones,” J. Physiol., 487, 1-16 (1995).
R. W. Tsien, and R. Y. Tsien, “Calcium channels, stores, and oscillations,” Annu. Rev. Cell Biol., 6, 715-760 (1990).
M. J. Berridge, “Inositol trisphosphate and calcium signalling,” Nature, 361, 315-325 (1993).
T. Nakamura, J. G. Barbara, K. Nakamura, and W. N. Ross, “Synergistic release of Ca2+ from IP3-sensitive stores evoked by synaptic activation of mGluRs paired with backpropagating action potentials,” Neuron, 24, 727-737 (1999).
E. Neher, and T. Sakaba, “Multiple roles of calcium ions in the regulation of neurotransmitter release,” Neuron, 59, 861-872 (2008).
C. Grienberger, and A. Konnerth, “Imaging calcium in neurons,” Neuron, 73, 862-885 (2012).
R. S. Zucker, “Calcium- and activity-dependent synaptic plasticity,” Curr. Opin. Neurobiol., 9, 305-313 (1999).
S. Koizumi, M. D. Bootman, L. K. Bobanovic et al., “Characterization of elementary Ca2+ release signals in NGF-differentiated PC12 cells and hippocampal neurons,” Neuron, 22, 125-137 (1999).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shkryl, V.M. Intracellular Calcium Fluxes in Excitable Cells. Neurophysiology 49, 384–392 (2017). https://doi.org/10.1007/s11062-018-9698-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11062-018-9698-2