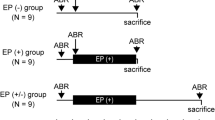
C57BL/6J mice develop age-related hearing loss (HL) early in life. The influence of aging and noise exposure on the number of presynaptic structures in inner hair cells of C57BL/6J mice has not been studied. We monitored auditory brainstem responses, ABRs, in C57BL/6J mice over time and assessed changes in the number of inner hair cell presynaptic ribbons. Multifaceted verification of the effects of aging and noise exposure on hearing in the C57BL/6J strain was performed. The HL was additively increased by noise exposure in 5-week-old mice. The earliest change observed was a decrease in the amplitude of the ABR first wave. Hair cells and spiral ganglion neurons were also lost. Immunohistochemistry and high-resolution confocal microscopy revealed decreased numbers of CtBP2-positive structures in the inner hair cells localized in other than those in the low-frequency region of the cochlea. On the other hand, the influence of acute noise exposure on the inner hair cell ribbons was observed only within the highest-frequency area.
Similar content being viewed by others
References
B. Yueh, N. Shapiro, C. H. MacLean, and P. G. Shekelle, “Screening and management of adult hearing loss in primary care: scientific review,” J. Am. Med. Assoc. 289, 1976-1985 (2003).
F. R. Lin, R. Thorpe, S. Gordon-Salant, and L. Ferrucci, “Hearing loss prevalence and risk factors among older adults in the United States,” J. Gerontol. Biol. Sci. Med. Sci., 66, 582-590 (2011).
S. G. Kujawa and M. C. Liberman, “Acceleration of agerelated hearing loss by early noise exposure: evidence of a misspent youth,” J. Neurosci. 26, 2115-2123 (2006).
E. Daniel, “Noise and hearing loss: a review,” J. School Health, 77, No. 5, 225-231 (2007).
D. O. Mikaelian, “Development and degeneration of hearing in the C57/b16 mouse: relation of electrophysiologic responses from the round window and cochlear nucleus to cochlear anatomy and behavioral responses,” Laryngoscope, 89, 1-15 (1979).
K. R. Henry and R. A. Chole, “Genotypic differences in behavioral, physiological and anatomical expressions of age-related hearing loss in the laboratory mouse,” Audiology, 19, 369-383 (1980).
J. F. Willott, J. Kulig, and T. Satterfield, “The acoustic startle response in DBA/2 and C57BL/6 mice: relationship to auditory neuronal response properties and hearing impairment,” Hear. Res., 16, 161-167 (1984).
J. F. Willott, “Effects of aging, hearing loss, and anatomical location on thresholds of inferior colliculus neurons in C57BL/6 and CBA mice,” J. Neurophysiol., 56, 391-408 (1986).
K. Parham and J. F. Willott, “Acoustic startle response in young and aging C57BL/6J and CBA/J mice,” Behav. Neurosci., 102, 881 (1988).
V. P. Spongr, D. G. Flood, R. D. Frisina, and R. J. Salvi, “Quantitative measures of hair cell loss in CBA and C57BL/6 mice throughout their life spans,” J. Acoust. Soc. Am., 101, 3546-3553 (1997).
R. R. Davis, J. K. Newlander, X. B. Ling, et al., “Genetic basis for susceptibility to noise-induced hearing loss in mice,” Hear. Res., 155, 82-90 (2001).
J. F. Willott, J. G. Turner, S. Carlson, et al., “The BALB/c mouse as an animal model for progressive sensorineural hearing loss,” Hear. Res., 115, 162-174 (1998).
L. C. Erway, J. F. Willott, J. R. Archer, and D. E. Harrison, “Genetics of age-related hearing loss in mice: I. Inbred and F1 hybrid strains,” Hear. Res., 65, 125-132 (1993).
K. Noben-Trauth, Q. Y. Zheng, and K. R. Johnson, “Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss,” Natl. Genet., 35, 21-23 (2003).
J. Siemens, C. Lillo, R. A. Dumont, et al., “Cadherin 23 is a component of the tip link in hair-cell stereocilia,” Nature, 428, 950-955 (2004).
S. P. Zachary and P. A. Fuchs, “Re-emergent inhibition of cochlear inner hair cells in a mouse model of hearing loss,” J. Neurosci., 35, No. 26, 9701-9706 (2015).
H. F. Schuknecht, The Pathology of the Ear, Harvard Univ. Press, Cambridge (1974).
H. S. Li and E. Borg, “Age-related loss of auditory sensitivity in two mouse genotypes,” Acta Otolaryngol., 111, 827-834 (1991).
S. Hequembourg and M. C. Liberman, “Spiral ligament pathology: a major aspect of age-related cochlear degeneration in C57BL/6 mice,” J. Assoc. Res. Otolaryngol., 2, 118-129 (2001).
M. C. Liberman, “Morphological differences among radial afferent fibers in the cat cochlea: an electronmicroscopic study of serial sections,” Hear. Res., 3, 45-63 (1980).
Y. Sergeyenko, K. Lall, M. C. Liberman, and S. G. Kujawa, “Age-related cochlear synaptopathy: an earlyonset contributor to auditory functional decline,” J. Neurosci., 33, 13686-13694 (2013).
A. C. Furman, S. G. Kujawa, and M. C. Liberman, “Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates,” J. Neurophysiol., 110, 577-586 (2013).
D. L. Newman, L. M. Fisher, J. Ohmen, et al., “GRM7 association with age-related hearing loss and its extension to additional features of presbycusis,” Hear. Res., 294, 125-132 (2012).
S. F. Maison, H. Usubuchi, and M. C. Liberman., “Efferent feedback minimizes cochlear neuropathy from moderate noise exposure,” J. Neurosci., 33, 5542-5552 (2013).
S. Stamataki, H. W. Francis, M. Lehar, et al., “Synaptic alterations at inner hair cells precede spiral ganglion cell loss in aging C57BL/6J mice,” Hear. Res., 221, 104-118 (2006).
H. J. G. Gundersen, “The nucleator,” J. Microscop., 151, 3-21 (1988).
A. Møller, P. Strange, and H. J. G. Gundersen, “Efficient estimation of cell volume and number using the nucleator and the disector,” J. Microscop., 159, 61-71 (1990).
T. Tandrup, “A method for unbiased and efficient estimation of number and mean volume of specified neuron subtypes in rat dorsal root ganglion,” J. Comp. Neurol., 329, 269-276 (1993).
F. Watanabe, M. Kirkegaard, S. Matsumoto, et al., “Signaling through erbB receptors is a critical functional regulator in the mature cochlea,” Eur. J. Neurosci., 32, 717-724 (2010).
P. Mannström, B. Ulfhake, M. Kirkegaard, and M. Ulfendahl, “Dietary restriction reduces age-related degeneration of stria vascularis in the inner ear of the rat,” Exp. Gerontol., 48, 1173-1179 (2013).
H. J. G. Gundersen, T. F. Bendtsen, L. Korbo, et al., “Some new, simple and efficient stereological methods and their use in pathological research and diagnosis,” Apmis, 96, 379-394 (1988).
H. J. G. Gundersen, P. Bagger, T. F. Bendtsen, et al., “The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis,” Apmis, 96, 857-881 (1988).
H. C. Ou, G. W. Harding, and B. A. Bohne, “An anatomically based frequency–place map for the mouse cochlea,” Hear. Res., 145, 123-129 (2000).
A. Viberg and B. Canlon, “The guide to plotting a cochleogram,” Hear. Res., 197, 1-10 (2004).
J. F. Willott, J. VandenBosche, T. Shimizu, et al., “Effects of exposing C57BL/6J mice to high-and lowfrequency augmented acoustic environments: Auditory brainstem response thresholds, cytocochleograms, anterior cochlear nucleus morphology and the role of gonadal hormones,” Hear. Res., 235, 60-71 (2008).
R. D. Frisina and X. Zhu, “Auditory sensitivity and the outer hair cell system in the CBA mouse model of agerelated hearing loss,” Open Access Anim. Physiol., 2, 9-16 (2010).
J. S. Buchwald and C. H. Huang, “Far-field acoustic response: origins in the cat,” Science, 189, 382-384 (1975).
S. G. Kujawa and M. C. Liberman, “Synaptopathy in the noise-exposed and aging cochlea: primary neural degeneration in acquired sensorineural hearing loss,” Hear. Res., 330, 191-199 (2015).
I. Tasaki, “The electrosaltatory transmission of the nerve impulse and the effect of narcosis upon the nerve fiber,” Am. J. Physiol., 127, 211-227 (1939).
N. Y. Kiang, M. C. Liberman, and R. A. Levine, “Auditory-nerve activity in cats exposed to ototoxic drugs and high-intensity sounds,” Ann. Otol. Rhinol. Laryngol., 85, 752-768 (1976).
H. Spoendlin, “Factors inducing retrograde degeneration of the cochlear nerve,” Ann. Otol. Rhinol. Laryngol., Suppl., 112, 76-82 (1983).
G. M. Cohen, J. C. Park, and J. S. Grasso, “Comparison of demyelination and neural degeneration in spiral and Scarpa’s ganglia of C57BL/6 mice,” J. Electron Microscop. Tech., 15, 165-172 (1990).
T. Kurioka, M. Y. Lee, A. N. Heeringa, et al., “Selective hair cell ablation and noise exposure lead to different patterns of changes in the cochlea and the cochlear nucleus,” Neuroscience, 332, 242-257 (2016).
H. Spoendlin, “Innervation patterns in the organ of Corti of the cat,” Acta Otolaryngol., 67, 239-254 (1969).
D. Robertson, “Functional significance of dendritic swelling after loud sounds in the guinea pig cochlea,” Hear. Res., 9, 263-278 (1983).
M. C. Liberman and N. Y. Kiang, “Acoustic trauma in cats: cochlear pathology and auditory-nerve activity,” Acta Otolaryngol., 358, 1-63 (1978).
A. M. Taberner and M. C. Liberman, “Response properties of single auditory nerve fibers in the mouse,” J. Neurophysiol., 93, 557-569 (2005).
R. A. Schmiedt, J. H. Mills, and F. A. Boettcher, “Agerelated loss of activity of auditory-nerve fibers,” J. Neurophysiol., 76, 2799-2803 (1996).
S. G. Kujawa and M. C. Liberman, “Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss,” J. Neurosci., 29, 14077-14085 (2009).
J. Bourien, Y. Tang, C. Batrel, et al., “Contribution of auditory nerve fibers to compound action potential of the auditory nerve,” J. Neurophysiol., 112, No. 5, 1025-1039 (2014).
H. W. Lin, A. C. Furman, S. G. Kujawa, and M. C. Liberman, “Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift,” J. Assoc. Res. Otolaryngol., 12, 605-616 (2011).
K. K. Ohlemiller, J. M. Lett, and P. M. Gagnon, “Cellular correlates of age-related endocochlear potential reduction in a mouse model,” Hear. Res., 220, 10-26 (2006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takeda, S., Mannström, P., Dash-Wagh, S. et al. Effects of Aging and Noise Exposure on Auditory Brainstem Responses and Number of Presynaptic Ribbons in Inner Hair Cells of C57BL/6J Mice. Neurophysiology 49, 316–326 (2017). https://doi.org/10.1007/s11062-018-9691-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11062-018-9691-9