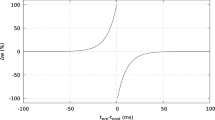
Spike timing-dependent plasticity (STDP) plays an important role in sculpting neural circuits to store information in the hippocampus, since motor learning and memory are thought to be closely linked with this type of synaptic plasticity. We built a computational model to study the potential learning rule by linearly changing the synaptic weight and number of the synapses involved. The main findings are the following: (i) changes in the synaptic weight and number of synapses can lead to different long-term changes in the synaptic efficacy; (ii) the first spike pair of two neurons exerts a great influence on the subsequent spike pair; a pre-post spiking pair reinforces the subsequent paired spiking, while a post-pre spiking pair depresses this paired spiking; (iii) when the synaptic weight and synaptic number change, the interval in the first spiking pair is reduced, which directly influences the first spiking pair, and (iv) when a stellate neuron is stimulated weakly or the capacitance of a CA1 pyramidal neuron is decreased, LTP is produced more easily than LTD; in the opposite case, LTD is produced more readily; an increase of the synaptic number can promote activation of CA1 pyramidal neurons.
Similar content being viewed by others
References
T. V. Bliss and T. Lomo, “Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path,” J. Physiol., 232, 331–356 (1973).
R. C. Malenka and S. A. Siegelbaum, Synaptic Plasticity, Johns Hopkins Univ. Press (2001).
S. J. Martin, P. D. Grimwood, and R. G. Morris, “Synaptic plasticity and memory: an evaluation of the hypothesis,” Annu. Rev. Neurosci., 23, 649–711 (2000).
Y. Dan and M. M. Poo, “Spike timing-dependent plasticity: from synapse to perception,” Physiol. Rev., 86, 1033–1048 (2006).
W. B. Levy and O. Steward, “Temporal contiguity requirements for long-term associative potentiation/depression in the hippocampus,” Neuroscience, 8, No. 4, 791–797 (1983).
G. Q. Bi and M. M. Poo, “Synaptic modifications in cultured hippocampal neurons: Dependence on spike timing, synaptic strength, and postsynaptic cell type,” J. Neurosci., 18, No. 24, 10464–10472 (1998).
C. Natalia and Y. Dan, “Spike timing-dependent plasticity: A hebbian learning rule,” Ann. Rev. Neurosci., 31, 25–46 (2008).
C. Vassilis, C. Stuart, and P. G. Bruce, “Encoding and retrieval in a model of the hippocampal CA1 microcircuit,” Hippocampus, 20, 423–446 (2010).
M. Royeck, M. T. Horstmann, S. Remy, et al., “Role of axonal NaV1.6 sodium channels in action potential initiation of CA1 pyramidal neurons,” J. Neurophysiol., 100, No. 4, 2361–2380 (2008).
H. Peter, E. Daniel, B. Angela, et al., “Distinct classes of pyramidal cells exhibit mutually exclusive firing patterns in hippocampal area CA3,” Hippocampus, 18, No. 4, 411–424 (2008).
W. M. Yamada, C. Koch, and P. R. Adams, Multiple Channels and Calcium Dynamics, MIT Press, Cambridge (1987), pp. 97–134
M. Migliore and G. M. Shepherd, “Dendritic action potentials connect distributed dendrodendritic microcircuits,” J. Comput. Neurosci., 24, 207–221 (2008).
R. Michel, M. T. Horstmann, R. Stefan, et al., “Role of axonal NaV 1.6 sodium channels in action potential initiation of CA1 pyramidal neurons,” J. Physiol., 4, 2361–2380 (2008).
L. Wang and S. Q. Liu, “Neural circuit and its functional roles in cerebellar cortex,” Neurosci. Bull., 27, No. 3, 173–184 (2011).
J. R. Hughes, “Post-tetanic potentiation,” Physiol. Rev., 38, No. 1, 91–113 (1958).
T. D. Joshua, T. David, and A. S. Steven, “A role for synaptic inputs at distal dendrites: Instructive signals for hippocampal long-term plasticity,” Neuron, 56, 866–879 (2007).
J. Tim, R. Alex, L. K. William, and S. Nelson, “Conditional dendritic spike propagation following distal synaptic activation of hippocampal CA1 pyramidal neurons,” Nat. Neurosci., 8, 1667–1676 (2005).
R. R. Clarke and J. R. Stephen, “Spatial segregation of neuronal calcium signals encodes different forms of LTP in rat hippocampus,” J. Physiol., 570, No. 1, 97–111 (2006).
T. Jarsky, A. Roxin, W. L. Kath, and N. Spruston, “Conditional dendritic spike propagation following distal synaptic activation of hippocampal CA1 pyramidal neurons,” Nat. Neurosci., 8, No. 12, 1667–1676 (2005).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ren, H., Liu, S.Q., Zhang, X. et al. Spike Timing-Dependent Plasticity in CA1 Pyramidal Neuron-Controlling Hippocampal Circuits: a Model Study. Neurophysiology 46, 300–307 (2014). https://doi.org/10.1007/s11062-014-9448-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11062-014-9448-z