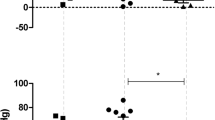
In acute experiments on rats anesthetized with urethane, features of the involvement of GABA in medullary cardiovascular control were studied. It was found that microinjections of GABA (10–8 or 10–10 M) into the medullary nuclei (paramedian reticular nucleus, PMn, lateral reticular nucleus, LRN, and nucl. ambiguous, AMB) were accompanied by the development of either hypo- or hypertensive responses in a dose-dependent manner. There were some differences in the structure of GABA-induced hemodynamic responses. In particular, the cardiac and vascular components contributed about equally to the development of the hypotensive responses caused by GABA injections into the PMn (with significant inhibition of the heart’s chronotropic function). However, GABA-induced hypotensive responses evoked from the LRN were mainly based on the vascular component, with the a less pronounced cardiac component. GABA injections into the AMB resulted in significant decreases in the diastolic blood pressure and the heart rate. As for GABA-induced hypertensive responses originated from PMn and LRN neurons, the vascular component was predominant in their development, and chronotropic effects on the cardiac function were less pronounced. Injections of bicuculline (10–7 M), a competitive antagonist of GABAA receptors, into the medullary nuclei under investigation were accompanied by increases in both the systolic and diastolic blood pressure and heart rate. Therefore, bicuculline-sensitive GABAA receptors are involved in GABA-induced hypotensive effects. After inhibition of neuronal NO synthase, injections of GABA into the medullary nuclei did not cause the development of hypotensive responses, and GABA-induced hypertensive responses were weakened, indicating the possibility for GABA interaction with nitric oxide in nervous control of the cardiovascular system. It was also found that the effects of GABA injected into the medullary nuclei depended on the activity of Na+,K+-ATPase, the enzyme of the plasma membrane of cardiovascular neurons.
Similar content being viewed by others
References
I A. Sytinskiy, Gamma-Aminobutyric Acid in the Nervous System Activity, Nauka, Leningrad, (1977)
G. E. Fagg and A. C. Foster, “Amino acid neurotransmitters and their pathways in the mammalian central nervous system,” Neuroscience, 9, No. 4, 701–719 (1983).
L.N. Shapoval and L.S. Pobegailo, “Changes in efferent activity in the renal nerve and vegetative reactions on administration of GABA in the structures of the ventrolateral medulla in cats,” Neirofiziologya/Neurophysiology, 19, No. 3, 327–334 (1987)
W. W. Blessing, “Depressor neurons in rabbit caudal medulla act via GABA receptors in rostral medulla,” Am. J. Physiol., 23, H786–H792 (1988).
R. N. Willette, A. J. Krieger, P. P. Barcas, and H. P. Sapru, “Medullary γ-aminobutyric acid (GABA) receptors and the regulation of blood pressure in the rat,” J. Pharmacol. Exp. Ther., 226, 893–899 (1983).
M. P. Meeley, D. A. Ruggiero, T. Ishitsuka, and D. J. Reis, “Intrinsic γ-aminobutyric acid neurons in the nucleus of the solitary tract and rostral ventrolateral medulla of the rat: immunocytochemical and biochemical study,” Neurosci. Lett., 58, 83–89 (1985).
M. Amano and T. Kubo, “Involvement of both GABAa and GABAb receptors in tonic inhibitory control of blood pressure at rostral ventrolateral medulla of the rat,” Naunyn-Schmiedeberg’s Arch. Pharmacol., 348, 146–153 (1993).
A. Milner, V. M. Pickel, J. Chan, et al., “Phenylethanolamine N-ethyl-transferase-containing neurons in the rostral ventrolateral medulla. II. Synaptic relationships with GABA-ergic terminals,” Brain Res., 411, 46–57 (1987).
L. N. Shapoval, V. F. Sagach, and L. S. Pobegailo, “Chemosensitive ventrolateral medulla in the cat: the fine structure and GABA-induced cardiovascular effects,” J. Auton. Nerv. Syst., 36, 159–172 (1991).
L. N. Shapoval, L.S. Pobeigailo, “Effect of GABA administrated in the medullary structures on the sympathetic activity and systemic arterial pressure level,” Fiziol. Zh. SSSR im. Sechenova, 68, No. 11, 1500–1505 (1982).
L. N. Shapoval, V. F. Sagach and L. S. Pobegailo, “Nitric oxide influences ventrolateral medullary mechanisms of vasomotor control in the cat,” Neurosci. Lett., 132, 47–50 (1991).
T. I. Krukoff, “Central actions of nitric oxide in 52–65 (1999).
S. Chowdhary and N. Townend, “Role of nitric oxide in the regulation of cardiovascular autonomic control,” Clin. Sci., 97, 5–17 (1999).
J. Zanzinger, “Role of nitric oxide in the neural control of cardiovascular functions,” Cardiovascul. Res., 43, 839–649 (1999).
L. N. Shapoval, V. F. Sagach, L. S. Pobegailo et al., “Involvement of nitric oxide in the medullary control of circulation in normotensive rats,” Neurophysiology, 34, No. 4, 294–302 (2002).
L. N. Shapoval, “Nitric oxide and nervous control of cardiovascular function,” in: Receptors, Channels and Messengers, P. G. Kostyuk and E. A. Lukyanetz, (eds.), DUS, Kiev (2005), pp. 318–337.
L. N. Shapoval, О. V. Dmytrenko, L. S. Pobegailo et al., “Hemodynamic responses induced by modulated nitric oxide system and mitochondrial permeability transition in the medullary cardiovascular neurons in rats,” Neurophysiology, 39, № 3, 232–244 (2007).
T. Kishi, Y. Hirooka, K. Sakai, et al., “Overexpression of eNOS in the RVLM causes hypertension and bradycardia via GABA release,” Hypertension, 38, No. 4, 896–904 (2001).
K. S. Rayevsky and V.P. Georgiev, Mediator Amino Acids: Neuropharmacological and Neurochemical Aspects, Meditsina, Moscow, Sofia (1986).
M. Chebib and G. A. R. Johnston, “GABA-activated ligand-gated ion channels: medical chemistry and molecular biology,” J. Med. Chem., 43, No. 8, 1427–1447 (2000).
A.A. Boldyrev, “The role of Na/K- pump in excitable tissues (review),” J. Sib. Fed. Univ. Biol., 3, 208–225 (2008).
J. B. Lingrel and T. Kuntzweiler, “Na/K-АТPase,” J. Biol. Chem., 269, 19659–19662 (1994).
K. Ikeda, H. Onimaru, J. Jamada, et al., “Malfunction of respiratory-related neuronal activity in Na+-K+-АТPase α2 subunit-deficient mice is attributable to abnormal Clhomeostasis in brainstem neurons,” J. Neurosci., 24, No. 47, 10693–10701 (2004).
G. Paxinos and C. Walson, The Rat Brain in Stereotaxic Coordinates, Academic Press, New York (1982).
D. R. Curtis, A. W. Duggan, D. Felix, and G. A. R. Johnston, “Bicuculline, an antagonist of GABA and synaptic inhibition in the spinal cord of the cat,” Brain Res., 32, 69–96 (1971)
A. S. Padilha, M. Salaices, S. D. V. Vassallo, et al., “Hypertensive effects of the i.v. administration of picomoles of ouabain,” Brazil. J. Med. Biol. Res., 44, 933–938 (2011).
M. Ferrandi, P. Barassi, I. Molinari, et al., “Ouabain antagonists as antihypertensive agents,” Curr. Pharm. Res., 11, No 25, 3301–3305 (2005).
X. Hou, S. T. Theriault, I. Dostanic-Larson, et al., “Enhanced pressor response to increased CSF sodium concentration and to central ANG 1 in heteroxygous α2 Na+-K+-АТPase knockout mice,” Am. J. Physiol. Regulat. Integr. Comp. Physiol., 296, R1427–R1438 (2009).
H. Takahashi, M. Yoshika, Yu. Komiyama, and M. Nishimura, “The central mechanism underlying hypertension: a review of the roles of sodium ions, epithelial sodium channels, the renin-angiotensin-aldosterone system, oxidative stress and endogenous digitalis in the brain,” Hypertens. Res., 34, 1147–1160 (2011).
J. C. Wang, J. A. Staessen, E. Messaggio, et al., “Salt, endogenous ouabain and blood pressure interactions in the general population,” J. Hypertens., 21, No. 8, 1475–1481 (2003).
J. Zhang, M. Y. Lee, M. Cavalli, et al., “Sodium pump alpha 2 subunits control myogenic tone and blood pressure in mice,” J. Physiol., 569, Part 1, 243–256 (2005).
B. S. Huang and F. H. Leenen, “Blockade of brain ‘ouabain’ prevents sympathoexcitatory and pressor responses to high sodium in SHR,” Am. J. Physiol., 271, Y103–H108 (1996).
A. Aydemir-Koksoy, J. Abramovitz, and J. C. Allen, “Ouabain-induced signaling and vascular smooth muscle cell proliferation,” J. Biol. Chem., 276, 46605–46611 (2001).
L. N. Shapoval, O.V. Dmytrenko, G.L. Vavilova et al. “Effect of modulation of Na+,K+-ATPase in the medullary cardiovascular neurons on hemodynamic effects in spontaneously hypertensive rats,” Fіzіol. Zh., 58, No 5, 3–13 (2012).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Radchenko, N.V., Shapoval, L.N., Davydovskaya, T.L. et al. Features of GABAergic Cardiovascular Control Provided by Medullary Neurons in Rats. Neurophysiology 45, 407–416 (2013). https://doi.org/10.1007/s11062-013-9386-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11062-013-9386-1