Abstract
Introduction
Maximal safe surgical resection followed by adjuvant chemoradiation has been standard for newly diagnosed glioblastoma multiforme (GBM). Hypofractionated accelerated radiotherapy (HART) has the potential to improve outcome as it reduces the overall treatment time and increases the biological effective dose.
Methods
Between October 2011 and July 2017, a total of 89 newly diagnosed GBM patients were randomized to conventional fractionated radiotherapy (CRT) or HART. Radiotherapy was delivered in all patients with a three-dimensional conformal radiotherapy technique in CRT arm (60 Gy in 30 fractions over 6 weeks @ 2 Gy/per fraction) or simultaneous integrated boost intensity modulated radiotherapy in HART arm (60 Gy in 20 fractions over 4 weeks @ 3 Gy/per fraction to high-risk planning target volume (PTV) and 50 Gy in 20 fractions over 4 weeks @ 2.5 Gy/per fraction to low-risk PTV). The primary endpoint of the trial was overall survival (OS).
Results
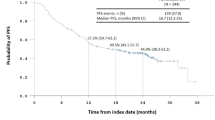
After a median follow-up of 11.4 months (Range: 2.9–42.5 months), 26 patients died and 39 patients had progression of the disease. Median OS for the entire cohort was 23.4 months. Median OS in the CRT and HART arms were 18.07 months (95% CI 14.52-NR) and 25.18 months (95% CI 12.89-NR) respectively, p = 0.3. Median progression free survival (PFS) for the entire cohort was 13.5 months (Range: 11.7–15.7 months). In multivariate analysis patients younger than 40 years of age, patients with a gross total resection of tumor and a mutated IDH-1 had significantly better OS. PFS was significantly better for patients with a gross total resection of tumor and a mutated IDH-1. All patients included in the trial completed the planned course of radiation. Only two patients required hospital admission for features of raised intracranial tension. One patient in the HART arm required treatment interruption.
Conclusion
HART is comparable to CRT in terms of survival outcome. HART arm had no excess treatment interruption and minimal toxicity. Dose escalation, reduction in overall treatment time, is the advantages with use of HART.




Similar content being viewed by others
References
Stupp R, Mason WP, van den Bent MJ, Weller M et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Stupp R, Hegi ME, Mason WP, van den Bent MJ et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10(5):434–435
Buglione M, Pedretti S, Poliani PL, Liserre R, Gipponi S, Spena G et al (2016) Pattern of relapse of glioblastoma multiforme treated with radical radio-chemotherapy: could a margin reduction be proposed? J Neurooncol 128(2):303–312
Minniti G, Amelio D, Amichetti M, Salvati M, Muni R, BozzaoA et al (2010) Patterns of failure and comparison of different target volume delineations in patients with glioblastoma treated with conformal radiotherapy plus concomitant and adjuvant temozolomide. Radiother Oncol 97(3):377–381
Lee JK, Chang N, Yoon Y, Yang H, Cho H, Kim E et al (2016) USP1 targeting impedes GBM growth by inhibiting stem cell maintenance and radioresistance. Neuro Oncol 18(1):37–47
Wick W, Platten M, Meisner C, Felsberg J, Tabatabai G, Simon M et al (2012) Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol 13(7):707–715
Malmström A, Grønberg BH, Marosi C, Stupp R, Frappaz D, Schultz H et al (2012) Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase III trial. Lancet Oncol 13(9):916–926
Roa W, Kepka L, Kumar N, Sinaika V, Matiello J, Lomidze D et al (2015) International Atomic Energy Agency randomized phase III study of radiation therapy in elderly and/or frail patients with newly diagnosed glioblastoma multiforme. J Clin Oncol 10(35):4145–4150 33
Cho KH, Kim JY, Lee SH, Yoo H, Shin SH, Moon SH et al (2010) Simultaneous integrated boost intensity-modulated radiotherapy in patients with high-grade gliomas. Int J Radiat Oncol Biol Phys 78(2):390–397
Panet-Raymond V, Souhami L, Roberge D, Kavan P, Shakibnia L, Muanza T et al (2009) Accelerated hypofractionated intensity-modulated radiotherapy with concurrent and adjuvant temozolomide for patients with glioblastomamultiforme: a safety and efficacy analysis. Int J Radiat Oncol Biol Phys 73(2):473–478
Iuchi T, Hatano K, Kodama T, Sakaida T, Yokoi S, Kawasaki K et al (2014) Phase II trial of hypofractionated high-dose intensity modulated radiation therapy with concurrent and adjuvant temozolomide for newly diagnosed glioblastoma. Int J Radiat Oncol Biol Phys 88:793–800
The National Cancer Institute (2009) Common Terminology Criteria for Adverse Events version 3.0 (CTCAE v3.0).
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28(11):1963–1972
Julka PK, Sharma DN, Mallick S, Gandhi AK, Joshi N, Rath GK (2013) Postoperative treatment of glioblastoma multiforme with radiation therapy plus concomitant and adjuvant temozolomide: a mono-institutional experience of 215 patients. J Cancer Res Ther 9(3):381–386
Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R et al (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 20(8):709–722 370
Gilbert MR1, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA et al (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370(8):699–708
Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA et al (2013) Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol 10(32):4085–4091 31
Stupp R, Hegi ME, Gorlia T, Erridge SC, Perry J, Hong YK et al (2014) Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071 – 22072 study): a multicentre, randomised, open-label, phase III trial. Lancet Oncol 15:1100–1108
Westphal M, Heese O, Steinbach JP, Schnell O, Schackert G, Mehdorn M et al (2015) A randomised, open label phase III trial with nimotuzumab, an anti-epidermal growth factor receptor monoclonal antibody in the treatment of newly diagnosed adult glioblastoma. Eur J Cancer 51:522–532
Reddy K, Damek D, Gaspar L, Ney D, Waziri A, Lillehei K et al (2012) Phase II trial of hypofractionated IMRT with temozolomide for patients with newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys 84(3):1–6
Ciammella P, Galeandro M, D’Abbiero N, Podgornii A, Pisanello A, Botti A et al (2013) Hypo-fractionated IMRT for patients with newly diagnosed glioblastoma multiforme: a 6 year single institutional experience. Clin Neurol Neurosurg 115(9):1609–1614
Amelio D, Lorentini S, Schwarz M, Amichetti M (2010) Intensity-modulated radiation therapy in newly diagnosed glioblastoma: a systematic review on clinical and technical issues. Radiother Oncol 97:361–369
Stummer W, Reulen HJ, Meinel T, Pichlmeier U et al. (2008) Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery 62:564–566
Louvel G, Metellus P, Noel G, Peeters S, Guyotat J, Duntze J et al (2016) Delaying standard combined chemoradiotherapy after surgical resection does not impact survival in newly diagnosed glioblastoma patients. Radiother Oncol 118(1):9–15
Lv L, Eda V, Callegaro-Filho S, Koch Lde D, Pontes Lde O, Weltman B (2016) E et al. Minimizing the uncertainties regarding the effects of delaying radiotherapy for Glioblastoma: a systematic review and meta-analysis. Radiother Oncol 118(1):1–8
Barker CA, Bishop AJ, Chang M, Beal K, Chan TA (2013) Valproic acid use during radiation therapy for glioblastoma associated with improved survival. Int J Radiat Oncol Biol Phys 86:504–509
Kerkhof M, Dielemans JC, van Breemen MS, Zwinkels H, Walchenbach R, Taphoorn MJ,et al (2013) Effect of valproic acid on seizure control and on survival in patients with glioblastoma multiforme. Neuro-oncology 15:961–967
Happold C, Gorlia T, Chinot O, Gilbert MR, Nabors LB, Wick W et al (2016) Does valproic acid or levetiracetam improve survival in glioblastoma? A pooled analysis of prospective clinical trials in newly diagnosed glioblastoma. J Clin Oncol 34(7):731–739
Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W et al (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360(8):765–773
Eckel-Passow JE, Lachance DH, Molinaro AM, Walsh KM, Decker PA, Sicotte H et al (2015) Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med 372(26):2499–2508
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mallick, S., Kunhiparambath, H., Gupta, S. et al. Hypofractionated accelerated radiotherapy (HART) with concurrent and adjuvant temozolomide in newly diagnosed glioblastoma: a phase II randomized trial (HART-GBM trial). J Neurooncol 140, 75–82 (2018). https://doi.org/10.1007/s11060-018-2932-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-2932-3