Abstract
Introduction
Pineal region glioblastomas (GBM) are very rare, with approximately 46 cases described in the literature. The epidemiology, pathogenesis, and treatment of these lesions are poorly characterized.
Methods
We identified all cases of pineal region GBM treated surgically at our institution between 1990 and 2017. Demographic and clinical follow-up data were extracted from the medical records for all cases. Pathology was reviewed and classified according to 2016 World Health Organization (WHO) criteria. Specific attention was given to the frequency of histone H3 K27M mutations in these midline gliomas.
Results
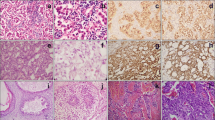
Eight patients (seven men, one woman) with pineal region GBM, WHO grade IV, were identified. The most common presenting symptoms were headache (75%), vision changes (75%), and gait imbalance/ataxia (50%). Median age at diagnosis was 48.5 years (range 36–74 years). Radical subtotal resection, via a supracerebellar infratentorial approach, was achieved in 75% of patients. Review of the surgical pathology revealed seven primary GBMs (including one giant cell GBM) and one pineal region GBM that developed three years after resection of a pineal parenchymal tumor of intermediate differentiation. No cases demonstrated evidence of IDH-1 R132H mutation (N = 6) or 1p/19q co-deletion (N = 3). One case tested positive for the histone H3 K27M-mutation. Targeted exome sequencing of 467 cancer-related genes revealed nonsense mutations in ATRX and NF1. Adjuvant radiation and chemotherapy was employed in 87.5% and 75.0% of patients, respectively. Median overall survival (OS) was 15 months (range 2–24 months) from GBM diagnosis.
Conclusions
This study expands the clinical and pathologic spectrum of pineal region GBM, and provides the first report of the genetic landscape of these tumors.


Similar content being viewed by others
Abbreviations
- GBM:
-
Glioblastoma
- WHO:
-
World Health Organization
- CNS:
-
Central nervous system
- PPTID:
-
Pineal parenchymal tumor of intermediate differentiation
- CUMC:
-
Columbia University Medical Center
- IRB:
-
Institutional Review Board
- MRI:
-
Magnetic resonance imaging
- r-STR:
-
Radical subtotal resection
- STR:
-
Subtotal resection
- OS:
-
Overall survival
- EBRT:
-
External beam radiotherapy
- SCIT:
-
Supracerebellar infratentorial
- IHTC:
-
Interhemispheric transcallosal
- COSMIC:
-
Catalogue of somatic mutations in cancer
- OMIM:
-
Online mendelian inheritance in man
- dbSNP:
-
Single nucleotide polymorphism database
References
Surawicz TS, McCarthy BJ, Kupelian V, Jukich PJ, Bruner JM, Davis FG (1999) Descriptive epidemiology of primary brain and CNS tumors: results from the Central Brain Tumor Registry of the United States, 1990–1994. Neuro Oncol 1(1):14–25
Nadvi SS, Timatkia K (2016) Primary pineal glioblastoma multiforme mimicking a germ cell tumour. Br J Neurosurg. https://doi.org/10.1080/02688697.2016.1265084
Matsuda R, Hironaka Y, Suigimoto T, Nakase H (2015) Glioblastoma multiforme in the pineal region with leptomeningeal dissemination and lumbar metastasis. J Korean Neurosurg Soc 58(5):479–482
Ringertz N, Nordenstam H, Flyger G (1954) Tumors of the pineal region. J Neuropathol Exp Neurol 13(4):540–561
Amini A, Schmidt RH, Salzman KL, Chin SS, Couldwell WT (2006) Glioblastoma multiforme of the pineal region. J Neurooncol 79(3):307–314
Birbilis TA, Matis GK, Eleftheriadis SG, Theodoropoulou EN, Sivridis E (2010) Spinal metastasis of glioblastoma multiforme: an uncommon suspect? Spine (Phila Pa 1976) 35(7):E264–E269
Bradfield JS, Perez CA (1972) Pineal tumors and ectopic pinealomas. Analysis of treatment and failures. Radiology 103(2):399–406
Cho BK, Wang KC, Nam DH et al (1998) Pineal tumors: experience with 48 cases over 10 years. Childs Nerv Syst 14(1–2):53–58
Edwards MS, Hudgins RJ, Wilson CB, Levin VA, Wara WM (1988) Pineal region tumors in children. J Neurosurg 68(5):689–697
Frank F, Gaist G, Piazza G, Ricci RF, Sturiale C, Galassi E (1985) Stereotaxic biopsy and radioactive implantation for interstitial therapy of tumors of the pineal region. Surg Neurol 23(3):275–280
Gasparetto EL, Warszawiak D, Adam GP, Bleggi-Torres LF, de Carvalho Neto A (2003) Glioblastoma multiforme of the pineal region: case report. Arq Neuropsiquiatr 61(2B):468–472
Kalyanaraman UP (1979) Primary glioblastoma of the pineal gland. Arch Neurol 36(11):717–718
Moon KS, Jung S, Jung TY, Kim IY, Lee MC, Lee KH (2008) Primary glioblastoma in the pineal region: a case report and review of the literature. J Med Case Rep 2:288
Norbut AM, Mendelow H (1981) Primary glioblastoma multiforme of the pineal region with leptomeningeal metastases: a case report. Cancer 47(3):592–596
Ozgural O, Kahilogullari G, Bozkurt M, Heper AO, Savas A (2013) Primary pineal glioblastoma: a case report. Turk Neurosurg 23(4):572–574
Pople IK, Arango JC, Scaravilli F (1993) Intrinsic malignant glioma of the pineal gland. Childs Nerv Syst 9(7):422–424
Toyooka T, Miyazawa T, Fukui S, Otani N, Nawashiro H, Shima K (2005) Central neurogenic hyperventilation in a conscious man with CSF dissemination from a pineal glioblastoma. J Clin Neurosci 12(7):834–837
Vaquero J, Ramiro J, Martinez R (1990) Glioblastoma multiforme of the pineal region. J Neurosurg Sci 34(2):149–150
Mansour J, Fields B, Macomson S, Rixe O (2014) Significant anti-tumor effect of bevacizumab in treatment of pineal gland glioblastoma multiforme. Target Oncol 9(4):395–398
DeGirolami U, Schmidek H (1973) Clinicopathological study of 53 tumors of the pineal region. J Neurosurg 39(4):455–462
Suzuki R, Suzuki K, Sugiura Y et al (2014) A case of glioblastoma multiforme in the pineal region with intraventricular hemorrhage. No Shinkei Geka 42(5):429–435
Liu Y, Hao S, Yu L, Gao Z (2015) Long-term temozolomide might be an optimal choice for patient with multifocal glioblastoma, especially with deep-seated structure involvement: a case report and literature review. World J Surg Oncol 13:142
Stowe HB, Miller CR, Wu J, Randazzo DM, Ju AW (2017) Pineal region glioblastoma, a case report and literature review. Front Oncol 7:123
Juan Y, et al (2010) Primary glioblastoma multiforme in the pineal region: a case report with diagnostic imaging findings, treatment response, and literature review. Chin J Radiol 35:115–123
Abecassis IJ, Hanak B, Barber J, Mortazavi M, Ellenbogen RG (2017) A single-institution experience with pineal region tumors: 50 tumors over 1 decade. Oper Neurosurg (Hagerstown) 13(5):566–575
Al-Tamimi YZ, Bhargava D, Surash S et al (2008) Endoscopic biopsy during third ventriculostomy in paediatric pineal region tumours. Childs Nerv Syst 24(11):1323–1326
Banczerowski P, Vajda J, Balint K, Sipos L (2012) Gliosarcoma of the pineal region with cerebellar metastasis: case illustration. Ideggyogy Sz 65(1–2):40–41
Bonney PA, Boettcher LB, Cheema AA, Maurer AJ, Sughrue ME (2015) Operative results of keyhole supracerebellar-infratentorial approach to the pineal region. J Clin Neurosci 22(7):1105–1110
Chang CG, Kageyama N, Kobayashi T, Yoshida J, Negoro M (1981) Pineal tumors: clinical diagnosis, with special emphasis on the significance of pineal calcification. Neurosurgery 8(6):656–668
Day GA, McPhee IB, Tuffley J et al (2007) Idiopathic scoliosis and pineal lesions in Australian children. J Orthop Surg (Hong Kong) 15(3):327–333
Jia W, Ma Z, Liu IY, Zhang Y, Jia G, Wan W (2011) Transcallosal interforniceal approach to pineal region tumors in 150 children. J Neurosurg Pediatr 7(1):98–103
Kumar P, Tatke M, Sharma A, Singh D (2006) Histological analysis of lesions of the pineal region: a retrospective study of 12 years. Pathol Res Pract 202(2):85–92
Luo SQ, Li DZ, Zhang MZ, Wang ZC (1989) Occipital transtentorial approach for removal of pineal region tumors: report of 64 consecutive cases. Surg Neurol 32(1):36–39
Oi S, Shibata M, Tominaga J et al (2000) Efficacy of neuroendoscopic procedures in minimally invasive preferential management of pineal region tumors: a prospective study. J Neurosurg 93(2):245–253
Pople IK, Athanasiou TC, Sandeman DR, Coakham HB (2001) The role of endoscopic biopsy and third ventriculostomy in the management of pineal region tumours. Br J Neurosurg 15(4):305–311
Sugita Y, Terasaki M, Tanigawa K et al (2016) Gliosarcomas arising from the pineal gland region: uncommon localization and rare tumors. Neuropathology 36(1):56–63
Thaher F, Kurucz P, Fuellbier L, Bittl M, Hopf NJ (2014) Endoscopic surgery for tumors of the pineal region via a paramedian infratentorial supracerebellar keyhole approach (PISKA). Neurosurg Rev 37(4):677–684
Orrego E, Casavilca S, Garcia-Corrochano P, Rojas-Meza S, Castillo M, Castaneda CA (2017) Glioblastoma of pineal region: report of four cases and literature review. CNS Oncol 6(4):251–259
Gilbert AR, Zaky W, Gokden M, Fuller CE, Ocal E, Leeds NE, Fuller GN (2018) Extending the neuroanatomic territory of diffuse midline glioma, K27M mutant: pineal region origin. Pediatr Neurosurg 53(1):59–63
Oliveira J, Cerejo A, Silva PS, Polónia P, Pereira J, Vaz R (2013 Nov) The infratentorial supracerebellar approach in surgery of lesions of the pineal region. Surg Neurol Int 30:4:154
Bruce JN, Ogden AT (2004) Surgical strategies for treating patients with pineal region tumors. J Neurooncol 69(1–3):221–236
Chandy MJ, Damaraju SC (1998) Benign tumours of the pineal region: a prospective study from 1983 to 1997. Br J Neurosurg 12(3):228–233
Kennedy BC, Bruce JN (2011) Surgical approaches to the pineal region. Neurosurg Clin N Am 22(3):367–380 viii.
Radovanovic I, Dizdarevic K, de Tribolet N, Masic T, Muminagic S (2009) Pineal region tumors–neurosurgical review. Med Arh 63(3):171–173
Sonabend AM, Bowden S, Bruce JN (2016) Microsurgical resection of pineal region tumors. J Neurooncol 130(2):351–366
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131(6):803–820
Khuong-Quang DA, Buczkowicz P, Rakopoulos P et al (2012) K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol 124(3):439–447
Wu G, Broniscer A, McEachron TA et al (2012) Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet 44(3):251–253
Solomon DA, Wood MD, Tihan T et al (2016) Diffuse midline gliomas with histone H3-K27M mutation: A series of 47 cases assessing the spectrum of morphologic variation and associated genetic alterations. Brain Pathol 26(5):569–580
Catalogue of Somatic Mutations in Cancer. http://www.cancersangeracuk/. 2017
Forbes SA, Beare D, Boutselakis H et al (2017) COSMIC: somatic cancer genetics at high-resolution. Nucleic Acids Res 45(D1):D777–D783
Louis DN, Ohgaki H, Wiestler OD et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114(2):97–109
Sanin V, Heess C, Kretzschmar HA, Schuller U (2013) Recruitment of neural precursor cells from circumventricular organs of patients with cerebral ischaemia. Neuropathol Appl Neurobiol 39(5):510–518
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Bruce JN, Stein BM (1995) Surgical management of pineal region tumors. Acta Neurochir (Wien) 134(3–4):130–135
D’Amico RS, Englander ZK, Canoll P, Bruce JN (2017) Extent of resection in glioma-a review of the cutting edge. World Neurosurg 103:538–549
Korshunov A, Ryzhova M, Hovestadt V et al (2015) Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathol 129(5):669–678
Sturm D, Witt H, Hovestadt V et al (2012) Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 22(4):425–437
Aihara K, Mukasa A, Gotoh K et al (2014) H3F3A K27M mutations in thalamic gliomas from young adult patients. Neuro Oncol 16(1):140–146
Feng J, Hao S, Pan C et al (2015) The H3.3 K27M mutation results in a poorer prognosis in brainstem gliomas than thalamic gliomas in adults. Hum Pathol 46(11):1626–1632
Morita S, Nitta M, Muragaki Y et al (2017) Brainstem pilocytic astrocytoma with H3 K27M mutation: case report. J Neurosurg. https://doi.org/10.3171/2017.4.JNS162443
Grasso CS, Tang Y, Truffaux N et al (2015) Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nat Med 21(6):555–559
Hashizume R, Andor N, Ihara Y et al (2014) Pharmacologic inhibition of histone demethylation as a therapy for pediatric brainstem glioma. Nat Med 20(12):1394–1396
Yu T, Sun X, Wang J, Ren X, Lin N, Lin S (2016) Twenty-seven cases of pineal parenchymal tumours of intermediate differentiation: mitotic count, Ki-67 labelling index and extent of resection predict prognosis. J Neurol Neurosurg Psychiatry 87(4):386–395
Jouvet A, Saint-Pierre G, Fauchon F et al (2000) Pineal parenchymal tumors: a correlation of histological features with prognosis in 66 cases. Brain Pathol 10(1):49–60
Fauchon F, Jouvet A, Paquis P et al (2000) Parenchymal pineal tumors: a clinicopathological study of 76 cases. Int J Radiat Oncol Biol Phys 46(4):959–968
Kim BS, Kim DK, Park SH (2009) Pineal parenchymal tumor of intermediate differentiation showing malignant progression at relapse. Neuropathology 29(5):602–608
Walsh KM, Wiencke JK, Lachance DH et al (2015) Telomere maintenance and the etiology of adult glioma. Neuro Oncol 17(11):1445–1452
Koschmann C, Calinescu AA, Nunez FJ et al (2016) ATRX loss promotes tumor growth and impairs nonhomologous end joining DNA repair in glioma. Sci Transl Med 8(328):328ra328
Vizcaino MA, Shah S, Eberhart CG, Rodriguez FJ (2015) Clinicopathologic implications of NF1 gene alterations in diffuse gliomas. Hum Pathol 46(9):1323–1330
Rodriguez FJ, Perry A, Gutmann DH et al (2008) Gliomas in neurofibromatosis type 1: a clinicopathologic study of 100 patients. J Neuropathol Exp Neurol 67(3):240–249
Cancer Genome Atlas Research N (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455(7216):1061–1068
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
D’Amico, R.S., Zanazzi, G., Wu, P. et al. Pineal region glioblastomas display features of diffuse midline and non-midline gliomas. J Neurooncol 140, 63–73 (2018). https://doi.org/10.1007/s11060-018-2931-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-2931-4