Abstract
Background
Recently, it has been shown that at group level, patients with limited brain metastases treated with stereotactic radiotherapy (SRT) maintain their pre-treatment levels of neurocognitive functioning (NCF) and health-related quality of life (HRQoL). The aim of this study was to evaluate NCF and HRQoL changes over time at the individual patient level.
Methods
NCF (seven domains assessed with a standardized test battery) and HRQoL (eight predetermined scales assessed with the EORTC QLQ-C30 and BN20 questionnaires) were measured prior to SRT and at 3 and/or 6 months follow-up. Changes in NCF and HRQoL were evaluated at (1) a domain/scale level and (2) patient level.
Results
A total of 55 patients were examined, of which the majority showed stable NCF 3 months after SRT, on both the domain level (78–100% of patients) and patient level (67% of patients). This was different for HRQoL, where deterioration in the different scales was observed in 12–61% of patients, stable scores in 20–71%, and improvement in 16–40%, 3 months after SRT. At patient level, most patients (64%) showed both improvement and deterioration in different HRQoL scales. Results were similar between 3 and 6 months after SRT.
Conclusion
In line with results at group level, most brain oligometastases patients with ≥ 6 months follow-up and treated with SRT maintained their pre-treatment level of NCF during this period. By contrast, changes in HRQoL scores differed considerably at domain and patient level, despite stable HRQoL scores at group level.
Similar content being viewed by others
Introduction
Brain metastases are a common manifestation of systemic cancer, with an estimated 9–45% of cancer patients developing brain metastases [1, 2]. The increasing incidence of brain metastases is most likely attributable to an aging population, the availability of improved imaging to detect smaller lesions, and better treatment modalities for systemic cancer which prolong life, thereby increasing the risk of dissemination of the systemic cancer to the brain [3, 4]. Although a subgroup of patients experiences longer survival [5], brain metastases are still incurable for most patients and the focus of treatment is mainly palliative [6]. Neurocognitive deficits and a reduced health-related quality of life (HRQoL) are often observed in patients with brain metastases and may be caused by the primary tumor, presence of (brain) metastases themselves, anti-tumor treatment, or supportive medication [4, 7,8,9,10]. Although survival is an important treatment endpoint for these patients, maintenance or improvement of neurocognitive functioning (NCF) and HRQoL during the course of the disease are at least as important [11, 12].
Whole-brain radiotherapy (WBRT) has been the standard of care in the past decades, but use of stereotactic radiotherapy (SRT) as an addition to, or as an alternative for, WBRT have increased considerably in recent years [13]. The main component of SRT is precise delivery of focal high dose radiation to a discrete target volume in 1–5 sessions, while minimizing irradiation of surrounding normal tissue [8, 14]. This treatment is particularly useful for patients presenting with limited brain metastases [15], which is the largest subgroup of patients, considering that 70% of the patients have three or fewer metastases [16]. SRT alone is associated with better NCF and HRQoL, while overall survival (OS) is comparable with WBRT alone or a combination of WBRT and SRT [5, 17, 18]. In contrast, SRT alone carries a risk of intracranial recurrences and patients treated with SRT undergo salvage treatment significantly more often compared with patients receiving both WBRT and SRT [19]. These salvage treatments may increase the risk of neurologic deficits and radionecrosis [4, 20].
Habets et al. [21] evaluated NCF and HRQoL prospectively in patients treated with SRT alone for 1–3 brain metastases and found that at group level, NCF and HRQoL remained relatively stable during 6 months from initial treatment, with the exception of physical functioning and fatigue, which worsened over time [21]. Although at group level patients maintained their pre-treatment levels of NCF and HRQoL to a large extent, this may not hold true for all individual patients. Since maintaining or improving NCF and HRQoL is important for all patients treated with SRT, we sought to evaluate changes over time in NCF and HRQoL at the individual patient level.
Materials and methods
Study population
Patients were eligible if they were ≥ 18 years; had ≤ 3 newly diagnosed brain metastases (maximum diameter of 4 cm); and were scheduled to undergo SRT, performed on an out-patient base with a dedicated Linac (Novalis; BrainLABAG, Helmstetten, Germany), construction year 2003. Recruitment of patients took place between January 2009 and February 2012. Exclusion criteria were: prior treatment for metastatic brain tumors; insufficient mastery of the Dutch language; and Karnofsky Performance Status (KPS) score < 70. The medical ethics committee of the institution approved the protocol. All patients provided written informed consent.
Procedures
The gross tumor volume (GTV) was contoured on a contrast-enhanced T1-weighted MRI. Planning target volume (PTV) was created by adding a 2-mm margin by 3D expansion to the clinical target volume (CTV), which was equal to the GTV. SRT treatment consisted of 21 Gy (PTV < 8 cm3) or 18 Gy (PTV 8–13 cm3) in a single fraction or 24 Gy (PTV > 13 cm3 and metastases near the brainstem) in three fractions of 8 Gy.
The baseline evaluation of NCF and HRQoL was conducted in the week preceding SRT. Follow-up assessments took place 3 and 6 months after SRT. Patients’ charts were examined to extract sociodemographic data and clinical variables, including primary tumor, treatment status and medication use. At all time points, MRI scans were made and the status of the primary disease and the use of medication were monitored. If patients showed intracranial progression during follow-up and underwent renewed SRT, provided the number of metastases was ≤ 3, these patients remained in the study. Patients with intracranial progression who transitioned to WBRT were excluded from further assessment. Patients were included in the statistical analysis if they complied for assessment on NCF and/or HRQoL on at least baseline and 3 months, or 3 and 6 months.
Study instruments
Neurocognitive functioning
NCF was assessed with a standardized battery of validated neurocognitive tests found to be clinically relevant in brain tumor patients (Supplementary Table 1) [22,23,24,25,26,27,28,29]. Individual test scores were combined in seven neurocognitive domain scores: verbal memory, visual memory, attention, executive functioning, working memory, information processing speed and visuoconstruction. Raw individual test scores were converted into standardized z-scores, by using means and standard deviations of individually matched healthy controls regarding age, gender, and education level, for four different domains (verbal memory, attention, executive functioning and information processing speed) [30,31,32]. Published norms were used, corrected for age and education, for the three other domains (visual memory, working memory and visuoconstruction) [33, 34]. A change in z-score of ≥ 1.5 standard deviation (SD) was considered to be clinically meaningful, in line with previous research in the same population [21].
Health-related quality of life
HRQoL was evaluated with two validated self-assessment questionnaires, (1) the for cancer patients developed 30-item generic European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30) and (2) the 20-item brain tumour-specific EORTC QLQ-Brain Cancer Module (QLQ-BN20) [35, 36]. A selection of HRQoL scales has been made, based on previous findings [37], comprising six QLQ-C30 scales (global health status, physical functioning, emotional functioning, role functioning, cognitive functioning, and fatigue) and two BN20 scales (motor dysfunction and communication deficits). Global health status was rated on a 7-point Likert scale, ranging from ‘very poor’ to ‘excellent’; the functioning and symptom scales were rated on a 4-point Likert scale, ranging from ‘not at all’ to ‘very much’. Raw scores were converted linearly into standardized scores ranging from 0 to 100. A higher score on the global health status and the functioning scales indicates better HRQoL, while on symptom-oriented scales a higher score indicates worse HRQoL. Difference or change score ≥ 10 points on any given scale were considered to be clinically meaningful [38].
Statistical analysis
To assess changes in HRQoL and NCF, differences in scores over time were calculated on (1) a domain/scale level and (2) patient level. Above described cut-off scores were used to determine an improvement, deterioration or stable score. Changes in scores were calculated for two different time periods: baseline-3 months, and 3–6 months. A cluster analysis, using R, was performed to identify whether specific HRQoL scales clustered.
Statistical analyses were performed using SPSS version 23.0. Statistical significance for intergroup differences were tested using the χ2 test for categorical variables, the Student’s t-tests or Mann–Whitney U-test for two-level continuous variables (depending on the distribution of the data), and the Kruskal–Wallis test for continuous variables with more than two levels. Kaplan–Meier curves were used for analyses of OS, and a log rank test to assess differences in survival. A p-value of < 0.05 was considered statistically significant.
Domain/scale level
For both time periods (baseline-3 months, and 3–6 months), patients were assigned to one of three categories: (1) deterioration, (2) stable score or (3) improvement, separately for each neurocognitive domain and HRQoL scale. For NCF, improvement and deterioration were defined as an increase or decrease in score ≥ 1.5 SD, respectively, and stable score as < 1.5 SD change. For HRQoL, improvement and deterioration were defined as ≥ 10 points increase or decrease respectively, and stable score as < 10 points increase or decrease.
Patient level
At patient level, patients were categorized into four categories, separately for NCF and HRQoL, applying the same cut-off scores as in the domain/scale level. These four categories were as follows: (1) decline, (2) improvement, (3) both and (4) stable. Decline and improvement were defined as deterioration or increase in NCF/HRQoL on at least one domain/scale respectively, while other domains/scales remained stable. The category ‘both’ included both a decline and improvement, whereas ‘stable’ was defined as no detectable change in any neurocognitive domain or HRQoL scale. Moreover, changes in KPS score, SRT dose received (biologically higher [single fraction 21 or 18 Gy] versus lower dosis [8 Gy in three fractions]), total tumor volume (as a proxy for GTV), intracranial progression and active systemic disease were assessed for the four categories, separately for the two time periods.
Results
Fifty-five out of the original 97 (57%) patients were eligible for analyses, because they had sufficient data. Baseline sociodemographic and clinical characteristics of the study population are summarized in Table 1. These baseline sociodemographic and clinical characteristics were compared between patients with and without sufficient NCF/HRQoL data. At baseline, patients without sufficient data had more often a lower KPS score (median of 80 [inter quartile range (IQR) = 70–80] vs. 80 [IQR = 80–90]; p = .002) and shorter OS (median of 3.8 months [IQR = 1.6–6.4] vs. 12.0 months [IQR = 8.2–12.0]; p < .001) when compared to patients with NCF/HRQoL data. NCF and HRQoL scores over time in our subpopulation were similar to the results as previously reported in the original study population (data not shown).
Patient characteristics
The mean age of the 55 included patients was 63 years (SD = 9) and the primary tumor was most frequently located in the lung (49%). Although the MRI scan showed a fourth metastasis in two patients, these patients received SRT because of the small size (< 0.5 cm3) and were therefore also included. The median total tumor volume at baseline was 7.3 cm3 (IQR = 3.4–12.8) and the 1-year survival rate was 48%, with all patients still alive after 3 months and 87% after 6 months from initial SRT.
Compliance
During follow-up, compliance dropped from 91% at baseline (n = 50) to 69% at 3 and 56% at 6 months for NCF, and from 98% at baseline (n = 54) to 93% at 3 and 85% at 6 months for HRQoL assessments (Supplementary Table 2). Patients had several reasons for non-compliance, such as progression of disease or the assessment being too demanding.
Neurocognitive functioning
Prior to SRT, half of the patients with neurocognitive data (25/50, 50%) showed impairments in at least one neurocognitive domain, of which verbal memory was most frequently affected (10/33, 30%).
Domain level
Three months after initial SRT, deterioration in the different neurocognitive domains was observed in 5/7 domains (3–8% of patients), while in two domains (verbal memory and visual memory) none of the patients showed deterioration (Fig. 1a). A stable score was observed in all domains (78–100% of patients), most frequently in verbal memory and visual memory. Improvement was found in 4/7 domains (3–17% of patients) and was most profound for visuoconstruction. Similar results were observed between 3 and 6 months after initial SRT [deterioration in 4/7 domains, 8–20% of patients; stable score in all domains, 73–100% of patients; and improvement in 3/7 domains, 4–8% of patients (Fig. 1b)]. Post-hoc analysis using a less stringent cut-off, a change in z-score of ≥ 1.0 SD, revealed similar results (Supplementary Fig. 1a; Supplementary Fig. 1b).
Patient level
Three months after initial SRT, 14% of patients showed a decline in NCF, another 14% an improvement, 6% both a decline and an improvement, while 67% had stable NCF (Fig. 1c). The period covering 3–6 months after initial SRT revealed similar results (decline 33%; improvement 13%; both 4%; and stable 50%). When using the ≥ 1.0 SD cut-off, scores differed from the original results, but still most patients remained stable or improved (Supplementary Fig. 1c).
Changes in KPS scores (Supplementary Table 3), SRT dose received, total tumor volume, intracranial progression or active systemic disease in individual patients did not differ significantly between the four different categories from baseline to 3 months or from 3 to 6 months after initial SRT.
Health-related quality of life
Prior to SRT, the vast majority (48/54, 89%) of patients showed a clinically relevant and statistically significant impairment in at least one of the six QLQ-C30 scales when compared to the general population (no reference data available for the QLQ-BN20 scores) [39], of which physical functioning was most frequently affected (31/54, 57%).
Scale level
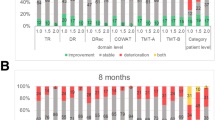
Three months after initial SRT, a decline in all eight different HRQoL scales was observed in 12–61% of patients, most often in fatigue (Fig. 2a). 20–71% of patients had stable scores and an improvement was shown in 16–40% of patients most frequently in communication deficit and motor dysfunction respectively. Comparable percentages were found between 3 and 6 months from initial SRT [deterioration 8–47% of patients; stable score 18–75% of patients; and improvement 11–34% of patients (Fig. 2b)].
Changes in health-related quality of life (HRQoL) scores calculated from a baseline-3 months and b 3–6 months at domain level and c patient level. GHS global health status; PF physical functioning; EF emotional functioning; RF role functioning; CF cognitive functioning; FA fatigue; MD motor dysfunction; CD communication deficits
Patient level
A decline in HRQoL in the first 3 months was observed in 22% of patients, an improvement in 12%, both worsening and improvement in 64%, while only 2% had a stable score (Fig. 2c).
Percentages were comparable 6 months after initial SRT (decline 21%; improvement 18%; both 58%; and stable 3%). Changes in KPS scores in individual patients differed significantly between the four categories from baseline to 3 months (p = .001) and from 3 to 6 months (p = .036) after initial SRT, with patients deteriorating in at least one HRQoL scale (in the ‘both’ and ‘decline’ category) showing most often worsening in performance status (Supplementary Table 3). No statistical significant differences between categories were found for SRT dose received, total tumor volume, intracranial progression or active systemic disease (data not shown).
Cluster analysis HRQoL
A heatmap was created to provide insight into changes in HRQoL at patient level (Fig. 3). The most striking pattern is that fatigue, and to a lesser extent emotional functioning, were clustered with global health status, indicating that a change on one scale is likely to be accompanied by a similar change on the other (i.e. decline, improve, both or remain stable). In addition, physical and role functioning were clustered, as well as several brain tumor-specific symptoms, these were motor dysfunction, communication deficit and self-perceived cognitive functioning.
Cluster analysis of differences in health-related-quality of life scores between a baseline-3 months and b 3–6 months. Black indicates deterioration; dark grey a stable score; light grey improvement; and white a missing value. On the vertical axis all 55 patients included in this study are displayed. a Patients are also clustered, but dendrogram is not shown. b Patients and HRQoL scales are similarly ordered for comparison. EF emotional functioning; FA fatigue; GHS global health status; PF physical functioning; RF role functioning; MD motor dysfunction; CD communication deficits; CF cognitive functioning
Discussion
The aim of this study was to evaluate changes in NCF and HRQoL at patient level 3 and 6 months after SRT, providing insight in the impact of treatment on the individual patient level. The overall results, in line with results at group level [21] and several other studies in patients with limited brain metastases [19, 40, 41], indicate that most patients with brain metastases treated with SRT maintained their pre-treatment levels of NCF for at least 6 months. Although NCF and HRQoL at the group level showed little variation, this is not necessarily translated into little variation at domain/scale and patient level. Indeed, changes in scores on the different HRQoL scales did vary substantially within patients, and most individual patients showed both a decline and improvement in separate HRQoL scales in the first 6 months after initial SRT. This finding is in contrast with the HRQoL findings at group level, in which patients who deteriorated and improved most likely cancelled each other out. When informing patients about the impact of a certain treatment or monitor their disease status, it is not sufficient to have information at group level only, nor at the scale level. Clinicians should also be aware that the large majority of patients will experience both deterioration and improvement in HRQoL.
An explanation for the relatively unaffected NCF in brain metastases patients may be that our study population represents a highly selected group of patients with good functioning. Indeed, patients in our sample had a higher KPS score and longer survival compared to our patients without sufficient NCF/HRQoL data. This is also supported by the finding that prior to SRT, only 50% of patients had an impairment in at least one neurocognitive domain, which is considerably lower than in previous studies in metastatic brain tumor patients (67–92%) [40, 42]. Particularly for neurocognitive testing, compliance rates decreased substantially over 6 months’ time. Responsible for non-compliance, among other things, were poor neurological or physical functioning and assessment considered too burdensome. Another explanation is the operational definition of objective neurocognitive decline, for which different cut-offs have been suggested [43]. Brown et al. [44], using a ≥ 1.0 SD cut-off score, found considerably higher neurocognitive deterioration rates compared to our study, with most patients showing cognitive deterioration at 3 months after SRT. However, when using a ≥ 1.0 SD cut-off in our study, still the majority of patients showed no cognitive deterioration, meaning a different cut-off does only partially explains the difference in neurocognitive deterioration rates [44]. Taking into account the aforementioned explanations for the relatively unaffected NCF, maintenance of NCF over 6 months’ time might have been overestimated in our biased sample and likely limits generalizability of the results to brain metastases patients with poor functioning.
Although average HRQoL remained stable at group level, except for physical functioning and fatigue, this did not hold true on scale level nor at patient level. On scale level, patients were relatively similarly distributed over the three different categories (deterioration; stable score; and improvement). At patient level, however, the majority of patients showed both deterioration and improvement in different HRQoL scales after radiotherapy, which has been previously reported in patients with brain metastases, but comparison is difficult because the majority of patients received WBRT instead of SRT [45, 46]. Caissie et al. [45] reported that upon follow-up 1 month after radiotherapy significant improvement was seen in several HRQoL scales, including communication deficit [45]. On the contrary, Steinmann et al. [46] reported that upon follow-up 3 months after the start of radiotherapy patients showed a significant and clinically relevant deterioration in several preselected HRQoL scales, including global health status, physical functioning, fatigue, motor dysfunction and communication deficit, while other scales remained unchanged [46]. In our study, the majority of patients showed a clinically relevant deterioration between baseline and 3 months in physical functioning (46%), role functioning (54%) and fatigue (61%), reflecting the findings at group level [21]. Nevertheless, considering the varying trajectories of changes in HRQoL after SRT, an important observation is that the majority of our patients showed both decline and improvement in separate HRQoL scales. An explanation for the varying trajectories of changes is that HRQoL measures vastly different concepts, encompassing physical, emotional, and social components, and that this outcome may be influenced by many factors, including comorbidity, marital status, heterogeneity of the primary tumor, SRT dose, total tumor volume, progression of the extracranial cancer and its corresponding supportive or anti-tumor treatment [47]. Although, SRT dose received, total tumor volume, intracranial progression and active systemic disease did not differ significantly between the four different categories at patient level, this result must be interpreted with caution due to our small sample size. As pointed out by Wilson and Cleary [48] in their model, more distal measures to the disease or the treatment (i.e. global health status and the functioning scales) are not only affected by health status but also by non-medical factors, as opposed to more proximal measures (i.e. symptoms) [48]. NCF is a proximal measure, which is mainly influenced by the presence of brain metastases, or its treatment. Patients who deteriorated on at least one HRQoL scale did most often have decreased performance status, suggesting that especially the patients’ overall functioning influences HRQoL. Moreover, Caissie et al. [49] found that fatigue and emotional functioning were the two strongest predictors of global health status in brain metastases patients, which is similar to the findings of our cluster analysis; deterioration in global health status clusters with increased fatigue and worse emotional functioning, suggesting fatigue may be a target for intervention to improve overall HRQoL [49].
To conclude, in accordance with previous results at group level, this study showed that most patients with brain oligometastases treated with SRT maintained their pre-treatment NCF for at least 6 months. However, changes in scores for the various HRQoL scales differed considerably between and within patients, suggesting that overall functioning is determined by complex underlying mechanisms which should be further analysed.
References
Schouten LJ, Rutten J, Huveneers HAM, Twijnstra A (2002) Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 94(10):2698–2705. https://doi.org/10.1002/cncr.10541
Barnholtz-Sloan JS, Yu CH, Sloan AE, Vengoechea J, Wang MH, Dignam JJ, Vogelbaum MA, Sperduto PW, Mehta MP, Machtay M, Kattan MW (2012) A nomogram for individualized estimation of survival among patients with brain metastasis. Neuro Oncol 14(7):910–918. https://doi.org/10.1093/neuonc/nos087
Nayak L, Lee EQ, Wen PY (2012) Epidemiology of brain metastases. Curr Oncol Rep 14(1):48–54. https://doi.org/10.1007/s11912-011-0203-y
Soffietti R, Ruda R, Trevisan E (2008) Brain metastases: current management and new developments. Curr Opin Oncol 20(6):676–684. https://doi.org/10.1097/CCO.0b013e32831186fe
Arvold ND, Lee EQ, Mehta MP, Margolin K, Alexander BM, Lin NU, Anders CK, Soffietti R, Camidge DR, Vogelbaum MA, Dunn IF, Wen PY (2016) Updates in the management of brain metastases. Neuro Oncol 18(8):1043–1065. https://doi.org/10.1093/neuonc/now127
Kamar FG, Posner JB (2010) Brain metastases. Semin Neurol 30(3):217–235. https://doi.org/10.1055/s-0030-1255225
Day J, Gillespie DC, Rooney AG, Bulbeck HJ, Zienius K, Boele F, Grant R (2016) Neurocognitive deficits and neurocognitive rehabilitation in adult brain tumors. Curr Treat Options Neurol. https://doi.org/10.1007/s11940-016-0406-5
Kaal ECA, Niël CGJH., Vecht CJ (2005) Therapeutic management of brain metastasis. Lancet Neurol 4(5):289–298. https://doi.org/10.1016/s1474-4422(05)70072-7
Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, Arbuckle RB, Swint JM, Shiu AS, Maor MH, Meyers CA (2009) Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 10(11):1037–1044. https://doi.org/10.1016/s1470-2045(09)70263-3
Pectasides D, Pectasides M, Economopoulos T (2006) Brain metastases from epithelial ovarian cancer: a review of the literature. Oncologist 11(3):252–260. https://doi.org/10.1634/theoncologist.11-3-252
Dirven L, Reijneveld JC, Taphoorn MJB (2014) Health-related quality of life or quantity of life: a difficult trade-off in primary brain tumors? Semin Oncol 41(4):541–552. https://doi.org/10.1053/j.seminoncol.2014.06.002
Schagen SB, Klein M, Reijneveld JC, Brain E, Deprez S, Joly F, Scherwath A, Schrauwen W, Wefel JS (2014) Monitoring and optimising cognitive function in cancer patients: present knowledge and future directions. EJC Suppl 12(1):29–40. https://doi.org/10.1016/j.ejcsup.2014.03.003
Muller-Riemenschneider F, Bockelbrink A, Ernst I, Schwarzbach C, Vauth C, von der Schulenburg JM, Willich SN (2009) Stereotactic radiosurgery for the treatment of brain metastases. Radiother Oncol 91(1):67–74. https://doi.org/10.1016/j.radonc.2008.12.001
Halasz LM, Rockhill JK (2013) Stereotactic radiosurgery and stereotactic radiotherapy for brain metastases. Surg Neurol Int 4(Suppl 4):S185–S191. https://doi.org/10.4103/2152-7806.111295
Sahgal A, Aoyama H, Kocher M, Neupane B, Collette S, Tago M, Shaw P, Beyene J, Chang EL (2015) Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 91(4):710–717. https://doi.org/10.1016/j.ijrobp.2014.10.024
Gavrilovic IT, Posner JB (2005) Brain metastases: epidemiology and pathophysiology. J Neurooncol 75(1):5–14. https://doi.org/10.1007/s11060-004-8093-6
Soliman H, Das S, Larson DA, Sahgal A (2016) Stereotactic radiosurgery (SRS) in the modern management of patients with brain metastases. Oncotarget 7(11):12318–12330. https://doi.org/10.18632/oncotarget.7131
Scoccianti S, Ricardi U (2012) Treatment of brain metastases: review of phase III randomized controlled trials. Radiother Oncol 102(2):168–179. https://doi.org/10.1016/j.radonc.2011.08.041
Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H, Kunieda E, Inomata T, Hayakawa K, Katoh N, Kobashi G (2006) Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases—a randomized controlled trial. JAMA 295(21):2483–2491. https://doi.org/10.1001/jama.295.21.2483
Regine WF, Huhn JL, Patchell RA, St Clair WH, Strottmann J, Meigooni A, Sanders M, Young AB (2002) Risk of symptomatic brain tumor recurrence and neurologic deficit after radiosurgery alone in patients with newly diagnosed brain metastases: results and implications. Int J Radiat Oncol Biol Phys 52(2):333–338. https://doi.org/10.1016/s0360-3016(01)02645-1
Habets EJJ, Dirven L, Wiggenraad RG, Verbeek-de Kanter A, Nijeholt G, Zwinkels H, Klein M, Taphoorn MJB (2016) Neurocognitive functioning and health-related quality of life in patients treated with stereotactic radiotherapy for brain metastases: a prospective study. Neuro Oncol 18(3):435–444. https://doi.org/10.1093/neuonc/nov186
Lezak MD, Howieson DB, Loring DW (2004) Neuropsychological assessment. Oxford University Press, New York
Tucha O, Smely C, Preier M, Lange KW (2000) Cognitive deficits before treatment among patients with brain tumors. Neurosurgery 47(2):324–333. https://doi.org/10.1097/00006123-200008000-00011
Saan RJ, Deelman BG (1986) De 15-woordentest A en B (een voorlopige handleiding) [The 15 words test A and B (a preliminary manual)]. Afdeling Neuropsychologie, AZG, Groningen
Uterwijk JMR (2000) WAIS-III Nederlandstalige bewerking. Technische handleiding. David Wechsler [WAIS-III Dutch revision. Technical manual. David Wechsler] Wechsler Adult Intelligence Scale III [Dutch revision. Technical manual]. Swets Test Publishers, Lisse
Van der Elst W, Van Boxtel MP, Van Breukelen GJ, Jolles J (2006) The concept shifting test: adult normative data. Psychol Assess 18(4):424. https://doi.org/10.1037/1040-3590.18.4.424
Krabbendam L, Kalff AC (1998) The behavioural assessment of the dysexecutive syndrome: Dutch version. Swets and Zeitlinger, Lisse
Meyers CA, Cantor SB (2003) Neuropsychological assessment and treatment of patients with malignant brain tumors. In: Prigatano GP, Pliskin NH (eds) Clinical neuropsychology and cost outcome research: a beginning. Psychology Press, New York, pp 159–173
Osterreith PA (1944) Le test de copie d’une figure complexe [A test regarding a complex figure]. Arch Psychol 30:206–356
Jolles J, van Boxtel MP, Ponds RW, Metsemakers JF, Houx PJ (1998) [The Maastricht aging study (MAAS). The longitudinal perspective of cognitive aging]. Tijdschr Gerontol Geriatr 29(3):120–129
De Bie SE (1987) Standaardvragen 1987: Voorstellen voor uniformering van vraagstellingen naar achtergrondkenmerken en interviews [Standard questions 1987: proposal for uniformisation of questions regarding background variables and interviews]. Leiden University Press, Leiden
Van der Elst W (2006) The neuropsychometrics of aging: normative studies in the Maastricht Aging Study. University of Maastricht, Maastricht
Fastenau PS, Denburg NL, Hufford BJ (1999) Adult norms for the Rey-Osterrieth complex figure test and for supplemental recognition and matching trials from the extended complex figure test. Clin Neuropsychol 13(1):30–47. https://doi.org/10.1076/clin.13.1.30.1976
Kessels RP, van den Berg E, Ruis C, Brands AM (2008) The backward span of the Corsi Block-Tapping Task and its association with the WAIS-III Digit Span. Assessment 15(4):426–434. https://doi.org/10.1177/1073191108315611
Osoba D, Aaronson NK, Muller M, Sneeuw K, Hsu MA, Yung WKA, Brada M, Newlands E (1996) The development and psychometric validation of a brain cancer quality-of-life questionnaire for use in combination with general cancer-specific questionnaires. Qual Life Res 5(1):139–150. https://doi.org/10.1007/bf00435979
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, Dehaes J, Kaasa S, Klee M, Osoba D, Razavi D, Rofe PB, Schraub S, Sneeuw K, Sullivan M, Takeda F (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85(5):365–376. https://doi.org/10.1093/jnci/85.5.365
Soffietti R, Kocher M, Abacioglu UM, Villa S, Fauchon F, Baumert BG, Fariselli L, Tzuk-Shina T, Kortmann RD, Carrie C, Ben Hassel M, Kouri M, Valeinis E, van den Berge D, Mueller RP, Tridello G, Collette L, Bottomley A (2013) A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol 31(1):65–72. https://doi.org/10.1200/jco.2011.41.0639
Osoba D, Rodrigues G, Myles J, Zee B, Pater J (1998) Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 16(1):139–144
van de Poll-Franse LV, Mols F, Gundy CM, Creutzberg CL, Nout RA, Verdonck-de Leeuw IM, Taphoorn MJ, Aaronson NK (2011) Normative data for the EORTC QLQ-C30 and EORTC-sexuality items in the general Dutch population. Eur J Cancer 47(5):667–675. https://doi.org/10.1016/j.ejca.2010.11.004
Chang EL, Wefel JS, Maor MH, Hassenbusch SJ III, Mahajan A, Lang FF, Woo SY, Mathews LA, Allen PK, Shiu AS, Meyers CA (2007) A pilot study of neurocognitive function in patients with one to three new brain metastases initially treated with stereotactic radiosurgery alone. Neurosurgery 60(2):277–283. https://doi.org/10.1227/01.neu.0000249272.64439.b1
Minniti G, Esposito V, Clarke E, Scaringi C, Bozzao A, Lanzetta G, De Sanctis V, Valeriani M, Osti M, Enrici RM (2013) Stereotactic radiosurgery in elderly patients with brain metastases. J Neurooncol 111(3):319–325. https://doi.org/10.1007/s11060-012-1016-z
Meyers CA, Smith JA, Bezjak A, Mehta MP, Liebmann J, Illidge T, Kunkler I, Caudrelier JM, Eisenberg PD, Meerwaldt J, Siemers R, Carrie C, Gaspar LE, Curran W, Phan SC, Miller RA, Renschler MF (2004) Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol 22(1):157–165. https://doi.org/10.1200/jco.2004.05.128
Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, Delis DC (2009) Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry 17(5):368–375. https://doi.org/10.1097/JGP.0b013e31819431d5
Brown PD, Jaeckle K, Ballman KV et al (2016) Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA 316(4):401–409. https://doi.org/10.1001/jama.2016.9839
Caissie A, Nguyen J, Chen E, Zhang L, Sahgal A, Clemons M, Kerba M, Arnalot PF, Danjoux C, Tsao M, Barnes E, Holden L, Danielson B, Chow E (2012) Quality of life in patients with brain metastases using the EORTC QLQ-BN20 + 2 and QLQ-C15-PAL. Int J Radiat Oncol Biol Phys 83(4):1238–1245. https://doi.org/10.1016/j.ijrobp.2011.09.025
Steinmann D, Paelecke-Habermann Y, Geinitz H, Aschoff R, Bayerl A, Bölling T, Bosch E, Bruns F, Eichenseder-Seiss U, Gerstein J, Gharbi N, Hagg J, Hipp M, Kleff I, Müller A, Schäfer C, Schleicher U, Sehlen S, Theodorou M, Wypior H-J, Zehentmayr F, van Oorschot B, Vordermark D (2012) Prospective evaluation of quality of life effects in patients undergoing palliative radiotherapy for brain metastases. BMC Cancer 12(1):283. https://doi.org/10.1186/1471-2407-12-283
Reeve BB, Potosky AL, Smith AW, Han PK, Hays RD, Davis WW, Arora NK, Haffer SC, Clauser SB (2009) Impact of cancer on health-related quality of life of older Americans. J Natl Cancer Inst 101(12):860–868. https://doi.org/10.1093/jnci/djp123
Wilson IB, Cleary PD (1995) Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA 273(1):59–65
Caissie A, Culleton S, Nguyen J, Zhang L, Zeng L, Holden L, Dennis K, Chan E, Jon F, Tsao M, Danjoux C, Sahgal A, Barnes E, Koo K, Chow E (2011) What QLQ-C15-PAL symptoms matter most for overall quality of life in patients with advanced cancer? World J Oncol 2(4):166–174
Funding
Funding was provided by St. Jacobusstichting, the Netherlands.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
van der Meer, P.B., Habets, E.J.J., Wiggenraad, R.G. et al. Individual changes in neurocognitive functioning and health-related quality of life in patients with brain oligometastases treated with stereotactic radiotherapy. J Neurooncol 139, 359–368 (2018). https://doi.org/10.1007/s11060-018-2868-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-2868-7