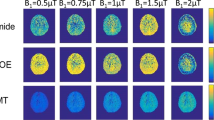
Abstract
Purpose
The objective was to investigate (with quantitative MRI) whether the normal appearing white matter (NAWM) of glioblastoma (GBM) patients on the contralateral side (cNAWM) was different from NAWM of healthy controls.
Methods
Thirteen patients with newly diagnosed GBM and nine healthy age-matched controls were MRI-scanned with quantitative magnetization transfer (qMT), chemical exchange saturation transfer (CEST), and transverse relaxation time (T2)-mapping. MRI scans were performed after surgery and before chemo-radiation treatment. Comprehensive qMT, CEST, T2 data were acquired. A two-pool MT model was fit to qMT data in transient state, to calculate MT model parameters \([{\text{R}},\,{{\text{T}}_{2{\text{b}}}},{\text{ R}}{{\text{M}}_{0{\text{b}}}}/{{\text{R}}_{\text{a}}},\,1/{{\text{R}}_{\text{a}}}{{\text{T}}_{2{\text{a}}}}]\). CEST signal was isolated by removing the contributions from the MT and direct water saturation, and CEST signal was calculated for Amide (CESTAmide), Amine (CESTAmine) and nuclear overhauser effect, NOE (CESTNOE).
Results
There was no difference between GBM patients and normal controls in the qMT properties of the macromolecular pool \(({\text{R}},\,{{\text{T}}_{2{\text{b}}}},{\text{ R}}{{\text{M}}_{0{\text{b}}}}/{{\text{R}}_{\text{a}}})\). However, their free water pool spectrum was different (1/RaT2a,patient = 28.1 ± 3.9, 1/RaT2a,control = 25.0 ± 1.1, p = 0.03). This difference could be attributed to the difference in their T2 time (\(T_{{2,\;{\text{patient}}}}^{{{\text{obs}}}}\) = 83 ± 4, \({T}_{{2,\;{\text{control}}}}^{{{\text{obs}}}}\) = 88 ± 1, p = 0.004). CEST signals were statistically significantly different with the CESTAmide having the largest difference between the two cohorts (CESTAmide,patient = 2.8 ± 0.4, CESTAmide,control = 3.4 ± 0.5, p = 0.009).
Conclusions
CEST in cNAWM of GBM patients was lower than healthy controls which could be caused by modified brain metabolism due to tumor cell infiltration. There was no difference in MT properties of the patients and controls, however, the differences in free water pool properties were mainly due to reduced T2 in cNAWM of the patients (resulting from structural changes and increased cellularity).




Similar content being viewed by others
References
Ryken TC, Aygun N, Morris J, Schweizer M, Nair R, Spracklen C, Kalkanis SN, Olson JJ (2014) The role of imaging in the management of progressive glioblastoma: a systematic review and evidence-based clinical practice guideline. J Neurooncol 118:435–460. https://doi.org/10.1007/s11060-013-1330-0
Easaw JC, Mason WP, Perry J, Laperrière N, Eisenstat DD, Del Maestro R, Bélanger K, Fulton D, Macdonald D (2011) Canadian recommendations for the treatment of recurrent or progressive glioblastoma multiforme. Curr Oncol 18(3). https://doi.org/10.3747/co.v18i3.755
Olson JJ, Ryken T (2008) Guidelines for the treatment of newly diagnosed glioblastoma: introduction. J Neurooncol 89:255–258. https://doi.org/10.1007/s11060-008-9595-4
Olson JJ, Fadul CE, Brat DJ, Mukundan S, Ryken TC (2009) Management of newly diagnosed glioblastoma: guidelines development, value and application. J Neuro-oncol 93:1–23. https://doi.org/10.1007/s11060-009-9838-z
Darefsky AS, King JTJ, Dubrow R (2012) Adult glioblastoma multiforme survival in the temozolomide era: a population-based analysis of Surveillance, Epidemiology, and End Results registries. Cancer 118:2163–2172. https://doi.org/10.1002/cncr.26494
Koshy M, Villano JL, Dolecek TA, Howard A, Mahmood U, Chmura SJ, Weichselbaum RR, McCarthy BJ (2012) Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J Neuro-oncol 107:207–212. https://doi.org/10.1007/s11060-011-0738-7
Cuddapah VA, Robel S, Watkins S, Sontheimer H (2014) A neurocentric perspective on glioma invasion. Nat Rev Neurosci 15:455–465. https://doi.org/10.1038/nrn3765
Dandy WE (1928) Removal of right cerebral hemisphere for certain tumors with hemiplegia: preliminary report. J Am Med Assoc 90:823–825. https://doi.org/10.1001/jama.1928.02690380007003
Sahm F, Capper D, Jeibmann A (2012) al et. Addressing diffuse glioma as a systemic brain disease with single-cell analysis. Arch Neurol 69:523–526
Scherer HJ (1938) Structural development in gliomas. Am J Cancer 34:333–351. https://doi.org/10.1158/ajc.1938.333
Giese A, Bjerkvig R, Berens ME, Westphal M (2003) Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol 21:1624–1636. https://doi.org/10.1200/JCO.2003.05.063
Giese A, Kluwe L, Laube B, Meissner H, Berens ME, Westphal M (1996) Migration of human glioma cells on myelin. Neurosurgery 38:755–764
Zhou J, Tryggestad E, Wen Z et al (2011) Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nat Med 17:130–134
Zaiss M, Windschuh J, Paech D et al (2015) Relaxation-compensated CEST-MRI of the human brain at 7T: unbiased insight into NOE and amide signal changes in human glioblastoma. Neuroimage 112:180–188. https://doi.org/10.1016/j.neuroimage.2015.02.040
Sagiyama K, Mashimo T, Togao O et al (2014) In vivo chemical exchange saturation transfer imaging allows early detection of a therapeutic response in glioblastoma. Proc Natl Acad Sci USA 111:4542–4547. https://doi.org/10.1073/pnas.1323855111
Ma B, Blakeley JO, Hong X et al (2016) Applying amide proton transfer-weighted MRI to distinguish pseudoprogression from true progression in malignant gliomas. J Magn Reson Imaging 44:456–462. https://doi.org/10.1002/jmri.25159
Mcvicar N, Li AX, Meakin SO, Bartha R (2015) Imaging chemical exchange saturation transfer (CEST) effects following tumor-selective acidification using lonidamine. NMR Biomed 28:566–575. https://doi.org/10.1002/nbm.3287
Heo HY, Zhang Y, Jiang S, Lee DH, Zhou J (2016) Quantitative assessment of amide proton transfer (APT) and nuclear overhauser enhancement (NOE) imaging with extrapolated semisolid magnetization transfer reference (EMR) signals: II. Comparison of three EMR models and application to human brain glioma at 4.7 tesla. Magn Reson Med 75:1630–1639. https://doi.org/10.1002/mrm.25795
Levesque IR, Giacomini PS, Narayanan S, Ribeiro LT, Sled JG, Arnold DL, Pike GB (2010) Quantitative magnetization transfer and myelin water imaging of the evolution of acute multiple sclerosis lesions. Magn Reson Med 63:633–640. https://doi.org/10.1002/mrm.22244
Horsfield MA (2005) Magnetization transfer imaging in multiple sclerosis. J Neuroimaging 15:58S–67S. https://doi.org/10.1177/1051228405282242
Tozer DJ, Rees JH, Benton CE, Waldman AD, Jäger HR, Tofts PS (2011) Quantitative magnetisation transfer imaging in glioma: preliminary results. NMR Biomed 24:492–498. https://doi.org/10.1002/nbm.1614
Newbould RD, Nicholas R, Thomas CL et al (2014) Age independently affects myelin integrity as detected by magnetization transfer magnetic resonance imaging in multiple sclerosis. NeuroImage 4:641–648. https://doi.org/10.1016/j.nicl.2014.02.004
Zhou J, Lal B, Wilson DA, Laterra J, Van Zijl PCM (2003) Amide proton transfer (APT) contrast for imaging of brain tumors. Magn Reson Med 50:1120–1126
Chavez S, Stanisz GJ (2012) A novel method for simultaneous 3D B1 and T1 mapping: the method of slopes (MoS). NMR Biomed 25:1043–1055
Chavez S (2012) Optimized method of slopes (MoS) produces robust and efficient 3D B1-corrected T1 maps. In: Proceedings of the international society for magnetic resonance in medicine, p. 2389
Chavez S (2015) A simple method (eMoS) for T1 mapping is more accurate and robust than the variable flip angle (VFA) method. In: Proceedings of the international society for magnetic resonance in medicine, p. 1673
Klein S, Staring M, Murphy K, Viergever MA, Pluim JPW (2010) elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging 29:196–205. https://doi.org/10.1109/TMI.2009.2035616
Henkelman RM, Huang X, Xiang QS, Stanisz GJ, Swanson SD, Bronskill MJ (1993) Quantitative interpretation of magnetization transfer. Magn Reson Med 29:759–766
Morrison C, Stanisz G, Henkelman RM (1995) Modeling magnetization transfer for biological-like systems using a semi-solid pool with a super-Lorentzian lineshape and dipolar reservoir. J Magn Reson B 108:103–113. https://doi.org/10.1006/jmrb.1995.1111
Mehrabian H, Myrehaug S, Soliman H, Sahgal A, Stanisz GJ (2018) Quantitative magnetization transfer in monitoring glioblastoma (GBM) response to therapy. Sci Rep 8:2475. https://doi.org/10.1038/s41598-018-20624-6
Stanisz G, Kecojevic A, Bronskill M, Henkelman R (1999) Characterizing white matter with magnetization transfer and T2. Magn Reson Med 42:1128–1136
Sled JG, Pike GB, Bruce Pike G (2001) Quantitative imaging of magnetization transfer exchange and relaxation properties in vivo using MRI. Magn Reson Med 46:923–931. https://doi.org/10.1002/mrm.1278
Portnoy S, Stanisz GJ (2007) Modeling pulsed magnetization transfer. Magn Reson Med 58:144–155. https://doi.org/10.1002/mrm.21244
Wu XZ, Listinsky JJ (1994) Effects of transverse cross relaxation on magnetization transfer. J Magn Reson B 105:73–76. https://doi.org/10.1006/jmrb.1994.1103
Listerud J (1997) Off-resonance pulsed magnetization transfer in clinical MR imaging: optimization by an analysis of transients. Magn Reson Med 37:693–705
Provotorov BN (1962) Magnetic resonance saturation in crystals. J Exp Theor Phys 14:1126–1131
Stanisz GJ, Odrobina EE, Pun J, Escaravage M, Graham SJ, Bronskill MJ, Henkelman RM (2005) T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn Reson Med 54:507–512. https://doi.org/10.1002/mrm.20605
Mehrabian H, Desmond KL, Soliman H, Sahgal A, Stanisz GJ (2017) Differentiation between radiation necrosis and tumor progression using chemical exchange saturation transfer. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-16-2265
Levesque IR, Stikov N, Pike GB, Pauly JM (2011) Drift in the magnetization transfer signal: effect on quantitative MT experiments. In: Proceedings of the international society for magnetic resonance in medicine, vol 19, p. 2782
Desmond KL, Moosvi F, Stanisz GJ (2014) Mapping of amide, amine, and aliphatic peaks in the CEST spectra of murine xenografts at 7T. Magn Reson Med 71:1841–1853
Windschuh J, Zaiss M, Meissner JE, Paech D, Radbruch A, Ladd ME, Bachert P (2015) Correction of B1-inhomogeneities for relaxation-compensated CEST imaging at 7T. NMR Biomed 28:529–537. https://doi.org/10.1002/nbm.3283
Desmond KL, Mehrabian H, Chavez S, Sahgal A, Soliman H, Rola R, Stanisz GJ (2016) Chemical exchange saturation transfer for predicting response to stereotactic radiosurgery in human brain metastasis. Magn Reson Med. https://doi.org/10.1002/mrm.26470
Cohen BA, Knopp EA, Rusinek H, Babb JS, Zagzag D, Gonen O (2005) Assessing global invasion of newly diagnosed glial tumors with whole-brain proton MR spectroscopy. Am J Neuroradiol 26:2170–2177
Gonen O, Viswanathan AK, Catalaa I, Babb J, Udupa J, Grossman RI (1998) Total brain N-acetylaspartate concentration in normal, age-grouped females: quantitation with non-echo proton NMR spectroscopy. Magn Reson Med 40:684–689
Horvath A, Perlaki G, Toth A, Orsi G, Nagy S, Doczi T, Horvath Z, Bogner P (2016) Biexponential diffusion alterations in the normal-appearing white matter of glioma patients might indicate the presence of global vasogenic edema. J Magn Reson Imaging 44:633–641. https://doi.org/10.1002/jmri.25202
Horvath A, Perlaki G, Toth A, Orsi G, Nagy S, Doczi T, Horvath Z, Bogner P (2016) Increased diffusion in the normal appearing white matter of brain tumor patients: is this just tumor infiltration? J Neuro-oncol 127:83–90. https://doi.org/10.1007/s11060-015-2011-y
Kallenberg K, Goldmann T, Menke J, Strik H, Bock HC, Mohr A, Buhk JH, Frahm J, Dechent P, Knauth M (2014) Abnormalities in the normal appearing white matter of the cerebral hemisphere contralateral to a malignant brain tumor detected by diffusion tensor imaging. Folia Neuropathol 52:226–233. https://doi.org/10.5114/fn.2014.45563
Inglese M, Brown S, Johnson G, Law M, Knopp E, Gonen O (2006) Whole-brain N-acetylaspartate spectroscopy and diffusion tensor imaging in patients with newly diagnosed gliomas: a preliminary study. Am J Neuroradiol 27:2137–2140
Togao O, Yoshiura T, Keupp J et al (2014) Amide proton transfer imaging of adult diffuse gliomas: correlation with histopathological grades. Neuro Oncol 16:441–448. https://doi.org/10.1093/neuonc/not158
Giese A, Loo MA, Tran N, Haskett D, Coons SW, Berens ME (1996) Dichotomy of astrocytoma migration and proliferation. Int J Cancer 67:275–282
Schiffer D, Cavalla P, Dutto A, Borsotti L (1997) Cell proliferation and invasion in malignant gliomas. Anticancer Res 17:61–69
Acknowledgements
This study was funded by Terry Fox Research Institute (TFRI project 1034), Canadian Cancer Society Research Institute (CCSRI 701640) and Brain Canada Grant (CCSRI 705083).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Mehrabian, H., Lam, W.W., Myrehaug, S. et al. Glioblastoma (GBM) effects on quantitative MRI of contralateral normal appearing white matter. J Neurooncol 139, 97–106 (2018). https://doi.org/10.1007/s11060-018-2846-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-2846-0