Abstract
Introduction
Concurrent radiotherapy and temozolomide (TMZ) is associated with radiographic pseudoprogression (PsP) in glioblastoma. The occurrence of PsP and other treatment effects is less well understood in low-grade gliomas (LGG). The purpose of this study is to evaluate whether the addition of TMZ to radiotherapy increases the incidence of PsP in adults with LGG treated with proton radiotherapy (PRT).
Methods
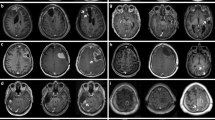
Chart review and volumetric MRI-analysis was performed on radiotherapy-naive adults with WHO grade II or IDH mutant WHO grade III gliomas treated with PRT between 2005 and 2015. Progression was defined by histology, new chemotherapy initiation, or progressive increase in lesion volume beyond 40% from baseline. Post treatment related effects (PTRE) were defined as new/increased T2/FLAIR or abnormal enhancement which eventually resolved or stabilized without evidence of progression for a period of 6–12 months. PsP was defined as the subset of PRTE suspicious for progression or volumetrically increased at least 40% from baseline. Pearson’s chi-squared test and Cox-proportional hazards models were used for statistical analysis.
Results
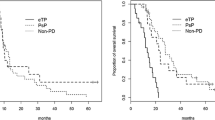
There were 119 patients meeting inclusion criteria. There was an increased risk of PsP following PRT + TMZ versus PRT-alone (HR = 2.2, p = 0.006, on Cox univariate analysis). Presence of PsP was associated with improved OS (p = 0.02 with PsP as time-varying covariate).
Conclusions
TMZ use, when added to PRT, was associated with increased PsP in patients with LGG; however, patients with PsP tended to achieve longer survival.



Similar content being viewed by others
References
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820
Smirniotopoulos JG, Murphy FM, Rushing EJ, Rees JH, Schroeder JW (2007) From the archives of the AFIP: patterns of contrast enhancement in the brain and meninges. Radiographics 27:525–551
Wen PY, Reardon DA (2016) Neuro-oncology in 2015: progress in glioma diagnosis, classification and treatment. Nat Rev Neurol 12:69
Van den Bent M, Smits M, Kros J, Chang S (2017) Diffuse infiltrating oligodendroglioma and astrocytoma. J Clin Oncol 35:2394–2394
Brat D, Verhaak R et al (2015) Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med 372:2481–2498
Kruser TJ, Mehta MP, Robins HI (2013) Pseudoprogression after glioma therapy: a comprehensive review. Expert Rev Neurother 13:389–403
Hoffman WF, Levin VA, Wilson CB (1979) Evaluation of malignant glioma patients during the postirradiation period. J Neurosurg 50:624–628
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Van den Bent MJ, Wefel JS, Schiff D et al (2011) Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol 12(6):583–593
Brandes AA, Franceschi E, Tosoni A et al (2008) MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol 26:2192–2197
Chamberlain MC, Glantz MJ, Chalmers L et al (2007) Early necrosis following concurrent temodar and radiotherapy in patients with glioblastoma. J Neurooncol 82(1):81–83
Taal W, Brandsma D, de Bruin HG et al (2008) Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer 113(2):405–410
Gerstner ER, McNamara MB, Norden AD, Lafrankie D, Wen PY (2009) Effect of adding temozolomide to radiation therapy on the incidence of pseudo-progression. J Neurooncol 94(1):97–101. https://doi.org/10.1007/s11060-009-9809-4
Gunjur A, Lau E, Taouk Y, Ryan G (2011) Early post-treatment pseudo-progression amongst glioblastoma multiforme patients treated with radiotherapy and temozolomide: a retrospective analysis. J Med Imaging Radiat Oncol 55:603–610
Kang H, Kim C, Han JH et al (2011) Pseudoprogression in patients with malignant gliomas treated with concurrent temozolomide and radiotherapy: potential role of p53. J Neuro-Oncol 102:157–162
Roldán GB, Scott JN, McIntyre JB et al (2009) Population-based study of pseudoprogression after chemoradiotherapy in GBM. Can J Neurol Sci 36:617–622
Sanghera P, Perry J, Sahgal A et al (2010) Pseudoprogression following chemoradiotherapy for glioblastoma multiforme. Can J Neurol Sci 37:36–42
Brandsma D et al (2008) Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 9:453–461
Langen K, Galldiks N, Hattingen E, Shah N (2017) Advances in neuro-oncology imaging. Nat Rev Neurol 13:279–289
Melguizo-Gavilanes I, Bruner JM, Guha-Thakurta N, Hess KR, Puduvalli VK (2015) Characterization of pseudoprogression in patients with glioblastoma: is histology the gold standard? J Neuro-Oncol 123:141–150
Hygino Da Cruz LC Jr, Rodriguez I, Domingues RC, Gasparetto EL, Sorensen AG (2011) Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. Am J Neuroradiol 32:1978–1985
Van West S, de Bruin H, van de Langerijt B, Swaak-Kragten A, van den Bent M, Taal W (2016) Incidence of pseudoprogression in low-grade gliomas treated with radiotherapy. Neuro-Oncology 19:719–725
De Wit MCY, De Bruin HG, Eijkenboom W, Sillevis Smitt PAE, Van Den Bent MJ (2004) Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology 63:535–537
Lin A, Liu J, Evans J et al (2014) Codeletions at 1p and 19q predict a lower risk of pseudoprogression in oligodendrogliomas and mixed oligoastrocytomas. Neuro-Oncology 16:123–130
Lin AL, White M, Miller-Thomas MM, Fulton RS, Tsien CI, Rich KM et al (2016) Molecular and histologic characteristics of pseudoprogression in diffuse gliomas. J Neurooncol 130:529–533
Meyzer C, Dhermain F, Ducreux D et al (2010) A case report of pseudoprogression followed by complete remission after proton-beam irradiation for a low-grade glioma in a teenager: the value of dynamic contrast-enhanced MRI. Radiat Oncol 5:9
Hauswald H, Rieken S, Swantje E, Kessel KA, Herfarth K, Debus J, Combs SE (2012) First experiences in treatment of low-grade glioma grade I and II with proton therapy. Radiat Oncol 7:189
Lassen-Ramshad Y, Petersen JB, Tietze A, Borghammer P, Mahajan A, McGovern SL (2015) Pseudoprogression after proton radiotherapy for pediatric low grade glioma. Acta Oncol 54(9):1701–1702 https://doi.org/10.3109/0284186X.2015.1078498
Naftel RP, Pollack IF, Zuccoli G, Deutsch M, Jakacki RI (2015) Pseudoprogression of low-grade gliomas after radiotherapy. Pediatr Blood Cancer 62:35–39
Bronk JK, Guha-Thakurta N, Allen PK, Mahajan A, Grosshans DR, McGovern SL (2018) Analysis of pseudoprogression after proton or photon therapy of 99 patients with low grade and anaplastic glioma. Clin Transl Radiat Oncol 9:30–34
Chappell R, Miranpuri SS, Mehta MP (1998) Dimension in defining tumor response. J Clin Oncol 16(3):1234
Ellingson BM, Wen PY, Cloughesy TF (2017) Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics 14:307–320
Motegi H, Kamoshima Y, Terasaka S, Kobayashi H, Yamaguchi S, Tanino M et al (2013) IDH1 mutation as a potential novel biomarker for distinguishing pseudoprogression from true progression in patients with glioblastoma treated with temozolomide and radiotherapy. Brain Tumor Pathol 30:67–72
McGovern SL, Okcu MF, Munsell MF, Kumbalasseriyil N, Grosshans DR, McAleer MF et al (2014) Outcomes and acute toxicities of proton therapy for pediatric atypical teratoid/rhabdoid tumor of the central nervous system. Int J Radiat Oncol Biol Phys 90:1143–1152
Gunther JR, Sato M, Chintagumpala M, Ketonen L, Jones JY, Allen PK et al (2015) Imaging changes in pediatric intracranial ependymoma patients treated with proton beam radiation therapy compared to intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys 93:54–63
Ruben JD, Dally M, Bailey M, Smith R, McLean CA, Fedele P (2006) Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys 65(2):499–508
Chawla S, Korones DN, Milano MT et al (2012) Spurious progression in pediatric brain tumors. J Neuro-Oncol 107:651–657
Bakardjiev AI, Barnes PD, Goumnerova LC et al (1996) Magnetic resonance imaging changes after stereotactic radiation therapy for childhood low grade astrocytoma. Cancer 78:864–873
Carceller F, Mandeville H, Mackinnon AD, Saran F (2017) Facing pseudoprogression after radiotherapy in low grade gliomas. Transl Cancer Res 6(Suppl 2):S254–S258
Carceller F, Fowkes LA, Khabra K et al (2016) Pseudoprogression in children, adolescents and young adults with non-brainstem high grade glioma and diffuse intrinsic pontine glioma. J Neurooncol 129:109–121
Clarke JL, Iwamoto FM, Sul J et al (2009) Randomized phase II trial of chemoradiotherapy followed by either dosedense or metronomic temozolomide for newly diagnosed glioblastoma. J Clin Oncol 27:3861–3867
Linhares P, Carvalho B, Figueiredo R et al (2013) Early pseudoprogression following chemoradiotherapy in glioblastoma patients: the value of RANO evaluation. J Oncol 2013:690585
MacDonald SM, Sethi R, Lavally B et al (2013) Proton radiotherapy for pediatric central nervous system ependymoma: clinical outcomes for 70 patients. Neuro Oncol 15:1552–1559
Indelicato DJ, Flampouri S, Rotondo RL et al (2014) Incidence and dosimetric parameters of pediatric brainstem toxicity following proton therapy. Acta Oncol 53:1298–1304
McGovern SL, Okcu MF, Munsell MF et al (2014) Outcomes and acute toxicities of proton therapy for pediatric atypical teratoid/rhabdoid tumor of the central nervous system. Int J Radiat Oncol Biol Phys 90:1143–1152
Giantsoudi D, Sethi RV, Yeap BY et al (2015) Incidence of CNS injury for a cohort of 111 patients treated with proton therapy for medulloblastoma: LET and RBE associations for areas of injury. Int J Radiat Oncol Biol Phys 95:287–296
Ares C, Albertini F, Frei-Welte M et al (2016) Pencil beam scanning proton therapy for pediatric intracranial ependymoma. J Neurooncol 128:137–145
Gentile MS, Yeap BY, Paganetti H et al (2018) Brainstem injury in pediatric patients with posterior fossa tumors treated with proton beam therapy and associated dosimetric factors. Int J Radiat Oncol Biol Phys 100(3):719–729
Fouladi M, Chintagumpala M, Laningham FH et al (2004) White matter lesions detected by magnetic resonance imaging after radiotherapy and high-dose chemotherapy in children with medulloblastoma or primitive neuroectodermal tumor. J Clin Oncol 22:4551–4560
Merchant TE, Li C, Xiong X et al (2009) Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol 10:258–266
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None of the authors have relevant disclosures.
Rights and permissions
About this article
Cite this article
Dworkin, M., Mehan, W., Niemierko, A. et al. Increase of pseudoprogression and other treatment related effects in low-grade glioma patients treated with proton radiation and temozolomide. J Neurooncol 142, 69–77 (2019). https://doi.org/10.1007/s11060-018-03063-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-03063-1