Abstract
Purpose
Fluorescence guided surgery by 5-aminolevulinic acid (5-ALA) and intraoperative MRI (iMRI) are currently the most important intraoperative imaging techniques in high grade glioma (HGG) surgery. Few comparative studies exist for these techniques. This review aims to systematically compare 5-ALA and iMRI assisted surgery based on the current literature and discuss the potential impact of a combined use of both techniques.
Methods
A systematic literature search based on preferred reporting items for systematic reviews and meta-analysis was performed concerning accuracy of tumor detection; extent of resection; neurological deficits (ND); Quality of life (QoL); usability and combined use of both techniques. Original clinical articles on HGG published until March 31st were screened.
Results
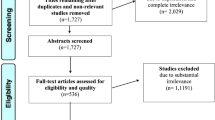
169 publications were screened, 81 were eligible and 22 were finally included in the review using. Overall, there is evidence that both imaging techniques improve gross total resection rate in non-eloquent lesions. Imaging results do not correlate at the border zone of a HGG. 5-ALA and contrast-enhanced iMRI seem to have a supplementary effect in tumor detection. Overall, both imaging techniques alone or combined do not seem to increase rate of permanent ND or decrease QoL in HGG surgery when used with intraoperative monitoring/mapping.
Conclusion
Based on the currently available literature no superiority of one technique over the other can be found in the most important outcome parameters. Based on the available information a combined use of 5-ALA and iMRI seems very promising to achieve a resection beyond gadolinium-enhancement. However, only low quality of evidence exists for this approach.


Similar content being viewed by others
References
Marko NF, Weil RJ, Schroeder JL, Lang FF, Suki D, Sawaya RE (2014) Extent of resection of glioblastoma revisited: personalized survival modeling facilitates more accurate survival prediction and supports a maximum-safe-resection approach to surgery. J Clin Oncol. https://doi.org/10.1200/jco.2013.51.8886
Sanai N, Berger MS (2008) Glioma extent of resection and its impact on patient outcome. Neurosurgery 62(4):753–764. https://doi.org/10.1227/01.neu.0000318159.21731.cf (discussion 264–756).
Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, Hess K, Michael C, Miller D, Sawaya R (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 95(2):190–198. https://doi.org/10.3171/jns.2001.95.2.0190
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen H-J (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7(5):392–401. https://doi.org/10.1016/s1470-2045(06)70665-9
Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V (2011) Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol 12(11):997–1003. https://doi.org/10.1016/s1470-2045(11)70196-6
Wu J-S, Gong X, Song Y-Y, Zhuang D-X, Yao C-J, Qiu T-M, Lu J-F, Zhang J, Zhu W, Mao Y, Zhou L-F (2014) 3.0-T Intraoperative magnetic resonance imaging-guided resection in cerebral glioma surgery: interim analysis of a prospective, randomized, triple-blind, parallel-controlled trial. Neurosurgery 61(CN_suppl_1):145–154. https://doi.org/10.1227/NEU.0000000000000372
Coburger J, Nabavi A, Konig R, Wirtz CR, Pala A (2017) Contemporary use of intraoperative imaging in glioma surgery: a survey among EANS members. Clin Neurol Neurosurg 163:133–141. https://doi.org/10.1016/j.clineuro.2017.10.033
Colditz MJ, Leyen K, Jeffree RL (2012) Aminolevulinic acid (ALA)-protoporphyrin IX fluorescence guided tumour resection. Part 2: theoretical, biochemical and practical aspects. J Clin Neurosci 19(12):1611–1616. https://doi.org/10.1016/j.jocn.2012.03.013
Stummer W, Tonn JC, Goetz C, Ullrich W, Stepp H, Bink A, Pietsch T, Pichlmeier U (2014) 5-Aminolevulinic acid-derived tumor fluorescence: the diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging. Neurosurgery 74(3):310–319. https://doi.org/10.1227/neu.0000000000000267 (discussion 319–320).
Ishihara R, Katayama Y, Watanabe T, Yoshino A, Fukushima T, Sakatani K (2007) Quantitative spectroscopic analysis of 5-aminolevulinic acid-induced protoporphyrin IX fluorescence intensity in diffusely infiltrating astrocytomas. Neurol Medico-chirurgica 47(2):53–57 (discussion 57)
Tonn JC, Stummer W (2008) Fluorescence-guided resection of malignant gliomas using 5-aminolevulinic acid: practical use, risks, and pitfalls. Clin Neurosurg 55:20–26
Nimsky C, Ganslandt O, Cerny S, Hastreiter P, Greiner G, Fahlbusch R (2000) Quantification of, visualization of, and compensation for brain shift using intraoperative magnetic resonance imaging. Neurosurgery 47(5):1070–1079 (discussion 1079–1080)
Wirtz CR, Tronnier VM, Bonsanto MM, Knauth M, Staubert A, Albert FK, Kunze S (1997) Image-guided neurosurgery with intraoperative MRI: update of frameless stereotaxy and radicality control. Stereotact Funct Neurosurg 68(1–4 Pt 1):39–43
Wirtz CR, Albert FK, Schwaderer M, Heuer C, Staubert A, Tronnier VM, Knauth M, Kunze S (2000) The benefit of neuronavigation for neurosurgery analyzed by its impact on glioblastoma surgery. Neurol Res 22(4):354–360
Nimsky C, Ganslandt O, Fahlbusch R (2005) Comparing 0.2 T with 1.5 T intraoperative magnetic resonance imaging analysis of setup, workflow, and efficiency. Acad Radiol 12(9):1065–1079. https://doi.org/10.1016/j.acra.2005.05.020
Coburger J, Merkel A, Scherer M, Schwartz F, Gessler F, Roder C, Pala A, Konig R, Bullinger L, Nagel G, Jungk C, Bisdas S, Nabavi A, Ganslandt O, Seifert V, Tatagiba M, Senft C, Mehdorn M, Unterberg AW, Rossler K, Wirtz CR (2016) Low-grade glioma surgery in intraoperative magnetic resonance imaging: results of a multicenter retrospective assessment of the German study group for intraoperative magnetic resonance imaging. Neurosurgery 78(6):775–786. https://doi.org/10.1227/NEU.0000000000001081
Roder C, Skardelly M, Ramina KF, Beschorner R, Honneger J, Nagele T, Tatagiba MS, Ernemann U, Bisdas S (2014) Spectroscopy imaging in intraoperative MR suite: tissue characterization and optimization of tumor resection. Int J Comput Assist Radiol Surg 9(4):551–559. https://doi.org/10.1007/s11548-013-0952-1
Roder C, Bender B, Ritz R, Honegger J, Feigl G, Naegele T, Tatagiba MS, Ernemann U, Bisdas S (2012) Intraoperative visualization of residual tumor: the role of perfusion-weighted imaging in a high-field intraoperative MR scanner. Neurosurgery 72:ons151–ons158
Javadi SA, Nabavi A, Giordano M, Faghihzadeh E, Samii A (2016) Evaluation of diffusion tensor imaging-based tractography of the corticospinal tract: a correlative study with intraoperative magnetic resonance imaging and direct electrical subcortical stimulation. Neurosurgery. https://doi.org/10.1227/neu.0000000000001347
Ostry S, Belsan T, Otahal J, Benes V, Netuka D (2013) Is intraoperative diffusion tensor imaging at 3.0T comparable to subcortical corticospinal tract mapping? Neurosurgery 73(5):797–807. https://doi.org/10.1227/neu.0000000000000087 (discussion 806–797).
Breitkopf M, Bisdas S, Liebsch M, Behling F, Bender B, Tatagiba M, Roder C (2017) Safety, utility, and clinical results of continuous intraoperative electrophysiological monitoring in 1.5T iMRI-guided surgery. World Neurosurg. https://doi.org/10.1016/j.wneu.2017.06.054
Pamir MN, Özduman K, Dinçer A, Yildiz E, Peker S, Özek MM (2010) First intraoperative, shared-resource, ultrahigh-field 3-Tesla magnetic resonance imaging system and its application in low-grade glioma resection. J Neurosurg 112(1):57–69. https://doi.org/10.3171/2009.3.JNS081139
Zhou Z, Lu Z-R (2013) Gadolinium-based contrast agents for MR cancer imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol 5(1):1–18. https://doi.org/10.1002/wnan.1198
Arbizu J, Tejada S, Marti-Climent JM, Diez-Valle R, Prieto E, Quincoces G, Vigil C, Idoate MA, Zubieta JL, Peñuelas I, Richter JA (2012) Quantitative volumetric analysis of gliomas with sequential MRI and 11C-methionine PET assessment: patterns of integration in therapy planning. Eur J Nucl Med Mol Imaging 39(5):771–781. https://doi.org/10.1007/s00259-011-2049-9
Yamahara T, Numa Y, Oishi T, Kawaguchi T, Seno T, Asai A, Kawamoto K (2010) Morphological and flow cytometric analysis of cell infiltration in glioblastoma: a comparison of autopsy brain and neuroimaging. Brain Tumor Pathol 27(2):81–87
Knauth M, Aras N, Wirtz CR, Dorfler A, Engelhorn T, Sartor K (1999) Surgically induced intracranial contrast enhancement: potential source of diagnostic error in intraoperative MR imaging. AJNR Am J Neuroradiol 20(8):1547–1553
Wirtz CR, Knauth M, Staubert A, Bonsanto MM, Sartor K, Kunze S, Tronnier VM (2000) Clinical evaluation and follow-up results for intraoperative magnetic resonance imaging in neurosurgery. Neurosurgery 46(5):1112–1120 (discussion 1120–1112)
Jenkinson MD, Barone DG, Bryant A, Vale L, Bulbeck H, Lawrie TA, Hart MG, Watts C (2018) Intraoperative imaging technology to maximise extent of resection for glioma. Cochrane Database Syst Rev 1:Cd012788. https://doi.org/10.1002/14651858.CD012788.pub2
Suero Molina E, Schipmann S, Stummer W (2017) Maximizing safe resections: the roles of 5-aminolevulinic acid and intraoperative MR imaging in glioma surgery-review of the literature. Neurosurg Rev. https://doi.org/10.1007/s10143-017-0907-z
Coburger J, Scheuerle A, Pala A, Thal D, Wirtz CR, König R (2017) Histopathological insights on imaging results of intraoperative magnetic resonance imaging, 5-aminolevulinic acid, and intraoperative ultrasound in glioblastoma surgery. Neurosurgery 81(1):165–174. https://doi.org/10.1093/neuros/nyw143
Coburger J, Engelke J, Scheuerle A, Thal DR, Hlavac M, Wirtz CR, Konig R (2014) Tumor detection with 5-aminolevulinic acid fluorescence and Gd-DTPA-enhanced intraoperative MRI at the border of contrast-enhancing lesions: a prospective study based on histopathological assessment. Neurosurg Focus 36(2):E3. https://doi.org/10.3171/2013.11.FOCUS13463
Roberts DW, Valdes PA, Harris BT, Fontaine KM, Hartov A, Fan X, Ji S, Lollis SS, Pogue BW, Leblond F, Tosteson TD, Wilson BC, Paulsen KD (2011) Coregistered fluorescence-enhanced tumor resection of malignant glioma: relationships between delta-aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. J Neurosurg 114(3):595–603
Hauser SB, Kockro RA, Actor B, Sarnthein J, Bernays RL (2016) Combining 5-aminolevulinic acid fluorescence and intraoperative magnetic resonance imaging in glioblastoma surgery: a histology-based evaluation. Neurosurgery 78(4):475–483. https://doi.org/10.1227/neu.0000000000001035
Idoate MA, Diez Valle R, Echeveste J, Tejada S (2011) Pathological characterization of the glioblastoma border as shown during surgery using 5-aminolevulinic acid-induced fluorescence. Neuropathology 31(6):575–582
Colditz MJ, Jeffree RL (2012) Aminolevulinic acid (ALA)-protoporphyrin IX fluorescence guided tumour resection. Part 1: clinical, radiological and pathological studies. J Clin Neurosci 19(11):1471–1474. https://doi.org/10.1016/j.jocn.2012.03.009
Schucht P, Knittel S, Slotboom J, Seidel K, Murek M, Jilch A, Raabe A, Beck J (2014) 5-ALA complete resections go beyond MR contrast enhancement: shift corrected volumetric analysis of the extent of resection in surgery for glioblastoma. Acta Neurochir (Wien) 156(2):305–312. https://doi.org/10.1007/s00701-013-1906-7 (discussion 312).
Arita H, Kinoshita M, Kagawa N, Fujimoto Y, Kishima H, Hashimoto N, Yoshimine T (2012) (1)(1)C-methionine uptake and intraoperative 5-aminolevulinic acid-induced fluorescence as separate index markers of cell density in glioma: a stereotactic image-histological analysis. Cancer 118(6):1619–1627
Floeth F, Sabel M, Ewelt C, Stummer W, Felsberg J, Reifenberger G, Steiger H, Stoffels G, Coenen H, Langen K-J (2011) Comparison of 18F-FET PET and 5-ALA fluorescence in cerebral gliomas. Eur J Nucl Med Mol Imaging 38(4):731–741. https://doi.org/10.1007/s00259-010-1690-z
Li YM, Suki D, Hess K, Sawaya R (2016) The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection? J Neurosurg 124(4):977–988. https://doi.org/10.3171/2015.5.jns142087
Barajas RF Jr, Phillips JJ, Parvataneni R, Molinaro A, Essock-Burns E, Bourne G, Parsa AT, Aghi MK, McDermott MW, Berger MS, Cha S, Chang SM, Nelson SJ (2012) Regional variation in histopathologic features of tumor specimens from treatment-naive glioblastoma correlates with anatomic and physiologic MR Imaging. Neuro-oncology 14(7):942–954
Diez Valle R, Slof J, Galvan J, Arza C, Romariz C, Vidal C (2014) Observational, retrospective study of the effectiveness of 5-aminolevulinic acid in malignant glioma surgery in Spain (The VISIONA study). Neurologia (Barcelona Spain) 29(3):131–138. https://doi.org/10.1016/j.nrl.2013.05.004
Schucht P, Beck J, Abu-Isa J, Andereggen L, Murek M, Seidel K, Stieglitz L, Raabe A (2012) Gross total resection rates in contemporary glioblastoma surgery: results of an institutional protocol combining 5-aminolevulinic acid intraoperative fluorescence imaging and brain mapping. Neurosurgery 71(5):927–936
Roder C, Bisdas S, Ebner FH, Honegger J, Naegele T, Ernemann U, Tatagiba M (2014) Maximizing the extent of resection and survival benefit of patients in glioblastoma surgery: high-field iMRI versus conventional and 5-ALA-assisted surgery. Eur J Surg Oncol 40(3):297–304. https://doi.org/10.1016/j.ejso.2013.11.022
Coburger J, Segovia von Riehm J, Ganslandt O, Wirtz CR, Renovanz M (2017) Is there an indication for intraoperative MRI in subtotal resection of glioblastoma? A multicenter retrospective comparative analysis. World Neurosurg. https://doi.org/10.1016/j.wneu.2017.11.015
Rahman M, Abbatematteo J, De Leo EK, Kubilis PS, Vaziri S, Bova F, Sayour E, Mitchell D, Quinones-Hinojosa A (2017) The effects of new or worsened postoperative neurological deficits on survival of patients with glioblastoma. J Neurosurg 127(1):123–131. https://doi.org/10.3171/2016.7.jns16396
McGirt MJ, Mukherjee D, Chaichana KL, Than KD, Weingart JD, Quinones-Hinojosa A (2009) Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery 65(3):463–469. https://doi.org/10.1227/01.neu.0000349763.42238.e9 (discussion 469–470).
Coburger J, Wirtz CR, Konig RW (2015) Impact of extent of resection and recurrent surgery on clinical outcome and overall survival in a consecutive series of 170 patients for glioblastoma in intraoperative high field iMRI. J Neurosurg Sci. https://doi.org/10.23736/S0390-5616.16.03284-7
Hatiboglu MA, Weinberg JS, Suki D, Rao G, Prabhu SS, Shah K, Jackson E, Sawaya R (2009) Impact of intraoperative high-field magnetic resonance imaging guidance on glioma surgery: a prospective volumetric analysis. Neurosurgery 64(6):1073–1081. https://doi.org/10.1227/01.neu.0000345647.58219.07 (discussion 1081).
Feigl GC, Ritz R, Moraes M, Klein J, Ramina K, Gharabaghi A, Krischek B, Danz S, Bornemann A, Liebsch M, Tatagiba MS (2010) Resection of malignant brain tumors in eloquent cortical areas: a new multimodal approach combining 5-aminolevulinic acid and intraoperative monitoring. J Neurosurg 113(2):352–357. https://doi.org/10.3171/2009.10.JNS09447
Stummer W, Tonn J-C, Mehdorn HM, Nestler U, Franz K, Goetz C, Bink A, Pichlmeier U (2011) Counterbalancing risks and gains from extended resections in malignant glioma surgery: a supplemental analysis from the randomized 5-aminolevulinic acid glioma resection study. J Neurosurg 114(3):613–623. https://doi.org/10.3171/2010.3.JNS097
Nickel K, Renovanz M, Konig J, Stockelmaier L, Hickmann AK, Nadji-Ohl M, Engelke J, Weimann E, Freudenstein D, Ganslandt O, Bullinger L, Wirtz CR, Coburger J (2017) The patients’ view: impact of the extent of resection, intraoperative imaging, and awake surgery on health-related quality of life in high-grade glioma patients-results of a multicenter cross-sectional study. Neurosurg Rev. https://doi.org/10.1007/s10143-017-0836-x
Coburger J, Nabavi A, König R, Wirtz CR, Pala A (2017) Contemporary use of intraoperative imaging in glioma surgery: a survey among EANS members. Clin Neurol Neurosurg 163(Supplement C):133–141. https://doi.org/10.1016/j.clineuro.2017.10.033
Coburger J, Scheuerle A, Pala A, Thal D, Wirtz CR, Konig R (2017) Histopathological insights on imaging results of intraoperative magnetic resonance imaging, 5-aminolevulinic acid, and intraoperative ultrasound in glioblastoma surgery. Neurosurgery 81(1):165–174. https://doi.org/10.1093/neuros/nyw143
Schatlo B, Fandino J, Smoll NR, Wetzel O, Remonda L, Marbacher S, Perrig W, Landolt H, Fathi AR (2015) Outcomes after combined use of intraoperative MRI and 5-aminolevulinic acid in high-grade glioma surgery. Neuro Oncol. https://doi.org/10.1093/neuonc/nov049
Gessler F, Forster M-T, Duetzmann S, Mittelbronn M, Hattingen E, Franz K, Seifert V, Senft C (2015) Combination of intraoperative magnetic resonance imaging and intraoperative fluorescence to enhance the resection of contrast enhancing gliomas. Neurosurgery 77(1):16–22. https://doi.org/10.1227/NEU.0000000000000729
Coburger J, Hagel V, Wirtz CR, Konig R (2015) Surgery for glioblastoma: impact of the combined use of 5-aminolevulinic acid and intraoperative MRI on extent of resection and survival. PLoS ONE 10(6):e0131872. https://doi.org/10.1371/journal.pone.0131872
Tsugu A, Ishizaka H, Mizokami Y, Osada T, Baba T, Yoshiyama M, Nishiyama J, Matsumae M (2011) Impact of the combination of 5-aminolevulinic acid-induced fluorescence with intraoperative magnetic resonance imaging-guided surgery for glioma. World Neurosurg 76(1–2):120–127. https://doi.org/10.1016/j.wneu.2011.02.005
Quick-Weller J, Lescher S, Forster MT, Konczalla J, Seifert V, Senft C (2016) Combination of 5-ALA and iMRI in re-resection of recurrent glioblastoma. Br J Neurosurg 30(3):313–317. https://doi.org/10.3109/02688697.2015.1119242
Potapov AA, Goryaynov SA, Okhlopkov VA, Shishkina LV, Loschenov VB, Savelieva TA, Golbin DA, Chumakova AP, Goldberg MF, Varyukhina MD, Spallone A (2016) Laser biospectroscopy and 5-ALA fluorescence navigation as a helpful tool in the meningioma resection. Neurosurg Rev 39(3):437–447. https://doi.org/10.1007/s10143-015-0697-0
Kamp MA, Grosser P, Felsberg J, Slotty PJ, Steiger HJ, Reifenberger G, Sabel M (2012) 5-aminolevulinic acid (5-ALA)-induced fluorescence in intracerebral metastases: a retrospective study. Acta Neurochir (Wien) 154(2):223–228. https://doi.org/10.1007/s00701-011-1200-5 (discussion 228).
Jaber M, Wolfer J, Ewelt C, Holling M, Hasselblatt M, Niederstadt T, Zoubi T, Weckesser M, Stummer W (2016) The value of 5-aminolevulinic acid in low-grade gliomas and high-grade gliomas lacking glioblastoma imaging features: an analysis based on fluorescence, magnetic resonance imaging, 18F-fluoroethyl tyrosine positron emission tomography, and tumor molecular factors. Neurosurgery 78(3):401–411. https://doi.org/10.1227/neu.0000000000001020 (discussion 411).
Coburger J, Konig R, Seitz K, Bazner U, Wirtz CR, Hlavac M (2014) Determining the utility of intraoperative magnetic resonance imaging for transsphenoidal surgery: a retrospective study. J Neurosurg 120(2):346–356. https://doi.org/10.3171/2013.9.JNS122207
Fahlbusch R, Ganslandt O, Buchfelder M, Schott W, Nimsky C (2001) Intraoperative magnetic resonance imaging during transsphenoidal surgery. J Neurosurg 95(3):381–390. https://doi.org/10.3171/jns.2001.95.3.0381
Pal’a A, Knoll A, Brand C, Etzrodt-Walter G, Coburger J, Wirtz CR, Hlavac M (2017) The value of intraoperative magnetic resonance imaging in endoscopic and microsurgical transsphenoidal pituitary adenoma resection. World Neurosurg 102:144–150. https://doi.org/10.1016/j.wneu.2017.02.132
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author JC works as a consultant for Brainlab AG, München, Germany and received speaker fees from the same company.
Rights and permissions
About this article
Cite this article
Coburger, J., Wirtz, C.R. Fluorescence guided surgery by 5-ALA and intraoperative MRI in high grade glioma: a systematic review. J Neurooncol 141, 533–546 (2019). https://doi.org/10.1007/s11060-018-03052-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-03052-4